Hypoxia regulates assembly of cilia in suppressors of Tetrahymena lacking an intraflagellar transport subunit gene
- PMID: 12925756
- PMCID: PMC181560
- DOI: 10.1091/mbc.e03-03-0166
Hypoxia regulates assembly of cilia in suppressors of Tetrahymena lacking an intraflagellar transport subunit gene
Abstract
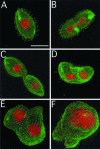
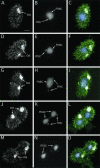
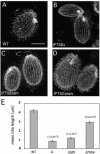
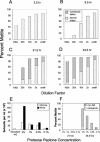
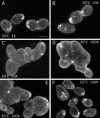
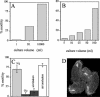
We cloned a Tetrahymena thermophila gene, IFT52, encoding a homolog of the Chlamydomonas intraflagellar transport protein, IFT52. Disruption of IFT52 led to loss of cilia and incomplete cytokinesis, a phenotype indistinguishable from that of mutants lacking kinesin-II, a known ciliary assembly transporter. The cytokinesis failures seem to result from lack of cell movement rather than from direct involvement of ciliary assembly pathway components in cytokinesis. Spontaneous partial suppressors of the IFT52 null mutants occurred, which assembled cilia at high cell density and resorbed cilia at low cell density. The stimulating effect of high cell density on cilia formation is based on the creation of pericellular hypoxia. Thus, at least under certain conditions, ciliary assembly is affected by an extracellular signal and the Ift52p function may be integrated into signaling pathways that regulate ciliogenesis.
Figures




References
-
- Brazelton, W.J., Amundsen, C.D., Silflow, C.D., and Lefebvre, P.A. (2001). The bld1 mutation identifies the Chlamydomonas osm-6 homolog as a gene required for flagellar assembly. Curr. Biol. 11, 1591–1594. - PubMed
-
- Brown, J.M., Hardin, C., and Gaertig, J. (1999a). Rotokinesis, a novel phenomenon of cell locomotion-assisted cytokinesis in the ciliate Tetrahymena thermophila. Int. Cell Biol. Rep. 23, 841–848. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

