SWAP-70 identifies a transitional subset of actin filaments in motile cells
- PMID: 12925760
- PMCID: PMC181564
- DOI: 10.1091/mbc.e03-01-0043
SWAP-70 identifies a transitional subset of actin filaments in motile cells
Abstract
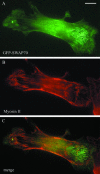
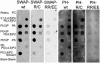
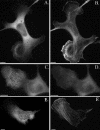
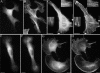
Functionally different subsets of actin filament arrays contribute to cellular organization and motility. We report the identification of a novel subset of loose actin filament arrays through regulated association with the widely expressed protein SWAP-70. These loose actin filament arrays were commonly located behind protruding lamellipodia and membrane ruffles. Visualization of these loose actin filament arrays was dependent on lamellipodial protrusion and the binding of the SWAP-70 PH-domain to a 3'-phosphoinositide. SWAP-70 with a functional pleckstrin homology-domain lacking the C-terminal 60 residues was targeted to the area of the loose actin filament arrays, but it did not associate with actin filaments. The C-terminal 60 residues were sufficient for actin filament association, but they provided no specificity for the subset of loose actin filament arrays. These results identify SWAP-70 as a phosphoinositide 3-kinase signaling-dependent marker for a distinct, hitherto unrecognized, array of actin filaments. Overexpression of SWAP-70 altered the actin organization and lamellipodial morphology. These alterations were dependent on a proper subcellular targeting of SWAP-70. We propose that SWAP-70 regulates the actin cytoskeleton as an effector or adaptor protein in response to agonist stimulated phosphatidylinositol (3,4)-bisphosphate production and cell protrusion.
Figures




Similar articles
-
Juxtanuclear Drebrin-Enriched Zone.Adv Exp Med Biol. 2017;1006:329-336. doi: 10.1007/978-4-431-56550-5_19. Adv Exp Med Biol. 2017. PMID: 28865029 Review.
-
The switch-associated protein 70 (SWAP-70) bundles actin filaments and contributes to the regulation of F-actin dynamics.J Biol Chem. 2013 Oct 4;288(40):28687-703. doi: 10.1074/jbc.M113.461277. Epub 2013 Aug 6. J Biol Chem. 2013. PMID: 23921380 Free PMC article.
-
DEF6, a novel PH-DH-like domain protein, is an upstream activator of the Rho GTPases Rac1, Cdc42, and RhoA.Exp Cell Res. 2004 Apr 1;294(2):335-44. doi: 10.1016/j.yexcr.2003.12.004. Exp Cell Res. 2004. PMID: 15023524
-
SWAP-70 regulates c-kit-induced mast cell activation, cell-cell adhesion, and migration.Mol Cell Biol. 2004 Dec;24(23):10277-88. doi: 10.1128/MCB.24.23.10277-10288.2004. Mol Cell Biol. 2004. PMID: 15542837 Free PMC article.
-
Assembling actin filaments for protrusion.Curr Opin Cell Biol. 2019 Feb;56:53-63. doi: 10.1016/j.ceb.2018.09.004. Epub 2018 Oct 1. Curr Opin Cell Biol. 2019. PMID: 30278304 Review.
Cited by
-
MiR-145: a potential biomarker of cancer migration and invasion.Am J Transl Res. 2019 Nov 15;11(11):6739-6753. eCollection 2019. Am J Transl Res. 2019. PMID: 31814885 Free PMC article. Review.
-
A mutant of SWAP-70, a phosphatidylinositoltrisphosphate binding protein, transforms mouse embryo fibroblasts, which is inhibited by sanguinarine.PLoS One. 2010 Dec 2;5(12):e14180. doi: 10.1371/journal.pone.0014180. PLoS One. 2010. PMID: 21152038 Free PMC article.
-
A screen for novel phosphoinositide 3-kinase effector proteins.Mol Cell Proteomics. 2011 Apr;10(4):M110.003178. doi: 10.1074/mcp.M110.003178. Epub 2011 Jan 24. Mol Cell Proteomics. 2011. PMID: 21263009 Free PMC article.
-
ASD-Associated De Novo Mutations in Five Actin Regulators Show Both Shared and Distinct Defects in Dendritic Spines and Inhibitory Synapses in Cultured Hippocampal Neurons.Front Cell Neurosci. 2018 Aug 3;12:217. doi: 10.3389/fncel.2018.00217. eCollection 2018. Front Cell Neurosci. 2018. PMID: 30123108 Free PMC article.
-
The Guanine Nucleotide Exchange Factor SWAP-70 Modulates the Migration and Invasiveness of Human Malignant Glioma Cells.Transl Oncol. 2009 Dec;2(4):300-9. doi: 10.1593/tlo.09172. Transl Oncol. 2009. PMID: 19956392 Free PMC article.
References
-
- Ballestrem, C., Wehrle-Haller, B., and Imhof, B.A. (1998). Actin dynamics in living mammalian cells. J. Cell Sci. 111, 1649–1658. - PubMed
-
- Borggrefe, T., Wabl, M., Akhmedov, A.T., and Jessberger, R. (1998). A B-cell-specific DNA recombination complex. J. Biol. Chem. 273, 17025–17035. - PubMed
-
- Borggrefe, T., Masat, L., Wabl, M., Riwar, B., Cattoretti, G., and Jessberger, R. (1999). Cellular, intracellular, and developmental expression patterns of murine SWAP-70. Eur. J. Immunol. 29, 1812–1822. - PubMed
-
- Burridge, K., and Chrzanowska-Wodnicka, M. (1996). Focal adhesions, contractility, and signaling. Annu. Rev. Cell. Dev. Biol. 12, 463–518. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

