Distinct mechanisms of agonist-induced endocytosis for human chemokine receptors CCR5 and CXCR4
- PMID: 12925765
- PMCID: PMC181569
- DOI: 10.1091/mbc.e02-11-0714
Distinct mechanisms of agonist-induced endocytosis for human chemokine receptors CCR5 and CXCR4
Abstract
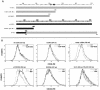
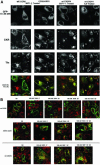
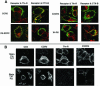
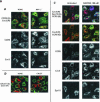
Desensitization of the chemokine receptors, a large class of G protein-coupled receptors, is mediated in part by agonist-driven receptor endocytosis. However, the exact pathways have not been fully defined. Here we demonstrate that the rate of ligand-induced endocytosis of CCR5 in leukocytes and expression systems is significantly slower than that of CXCR4 and requires prolonged agonist treatment, suggesting that these two receptors use distinct mechanisms. We show that the C-terminal domain of CCR5 is the determinant of its slow endocytosis phenotype. When the C-tail of CXCR4 was exchanged for that of CCR5, the resulting CXCR4-CCR5 (X4-R5) chimera displayed a CCR5-like trafficking phenotype. We found that the palmitoylated cysteine residues in this domain anchor CCR5 to plasma membrane rafts. CXCR4 and a C-terminally truncated CCR5 mutant (CCR5-KRFX) lacking these cysteines are not raft associated and are endocytosed by a clathrin-dependent pathway. Genetic inhibition of clathrin-mediated endocytosis demonstrated that a significant fraction of ligand-occupied CCR5 trafficked by clathrin-independent routes into caveolin-containing vesicular structures. Thus, the palmitoylated C-tail of CCR5 is the major determinant of its raft association and endocytic itineraries, differentiating it from CXCR4 and other chemokine receptors. This novel feature of CCR5 may modulate its signaling potential and could explain its preferential use by HIV for person-to-person transmission of disease.
Figures







References
-
- Alkhatib, G., Locati, M., Kennedy, P.E., Murphy, P.M., and Berger, E.A. (1997). HIV-1 coreceptor activity of CCR5 and its inhibition by chemokines: independence from G protein signaling and importance of coreceptor downmodulation. Virology 234, 340–348. - PubMed
-
- Alonso, M.A., and Millan, J. (2001). The role of lipid rafts in signalling and membrane trafficking in T lymphocytes. J. Cell Sci. 114, 3957–3965. - PubMed
-
- Amara, A., Gall, S.L., Schwartz, O., Salamero, J., Montes, M., Loetscher, P., Baggiolini, M., Virelizier, J.L., and Arenzana-Seisdedos, F. (1997). HIV coreceptor downregulation as antiviral principle: SDF-1alpha-dependent internalization of the chemokine receptor CXCR4 contributes to inhibition of HIV replication. J. Exp. Med. 186, 139–146. - PMC - PubMed
-
- Anderson, R.G. (1998). The caveolae membrane system. Annu. Rev. Biochem. 67, 199–225. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

