Subcellular localization and activity of multidrug resistance proteins
- PMID: 12925771
- PMCID: PMC181575
- DOI: 10.1091/mbc.e02-11-0704
Subcellular localization and activity of multidrug resistance proteins
Abstract
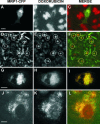
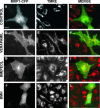
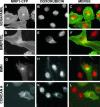
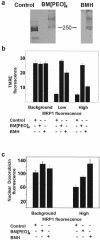
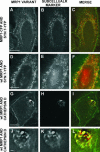
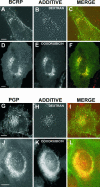
The multidrug resistance (MDR) phenotype is associated with the overexpression of members of the ATP-binding cassette family of proteins. These MDR transporters are expressed at the plasma membrane, where they are thought to reduce the cellular accumulation of toxins over time. Our data demonstrate that members of this family are also expressed in subcellular compartments where they actively sequester drugs away from their cellular targets. The multidrug resistance protein 1 (MRP1), P-glycoprotein, and the breast cancer resistance protein are each present in a perinuclear region positive for lysosomal markers. Fluorescence-activated cell sorting analysis suggests that these three drug transporters do little to reduce the cellular accumulation of the anthracycline doxorubicin. However, whereas doxorubicin enters cells expressing MDR transporters, this drug is sequestered away from the nucleus, its subcellular target, in vesicles expressing each of the three drug resistance proteins. Using a cell-impermeable inhibitor of MRP1 activity, we demonstrate that MRP1 activity on intracellular vesicles is sufficient to confer a drug resistance phenotype, whereas disruption of lysosomal pH is not. Intracellular localization and activity for MRP1 and other members of the MDR transporter family may suggest different strategies for chemotherapeutic regimens in a clinical setting.
Figures

References
-
- Cabrita, M.A., Hobman, T.C., Hogue, D.L., King, K.M., and Cass, C.E. (1999). Mouse transporter protein, a membrane protein that regulates cellular multidrug resistance, is localized to lysosomes. Cancer Res. 59, 4890–4897. - PubMed
-
- Chang, X.B., Hou, Y.X., and Riordan, J.R. (1997). ATPase activity of purified multidrug resistance-associated protein. J. Biol. Chem. 272, 30962–30968. - PubMed
-
- Chen, Y., Schindler, M., and Simon, S.M. (1999). A mechanism for tamoxifen-mediated inhibition of acidification. J. Biol. Chem. 274, 18364–18373. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

