M-CSF induces vascular endothelial growth factor production and angiogenic activity from human monocytes
- PMID: 12928417
- PMCID: PMC6905182
- DOI: 10.4049/jimmunol.171.5.2637
M-CSF induces vascular endothelial growth factor production and angiogenic activity from human monocytes
Abstract
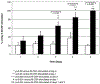
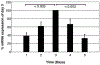
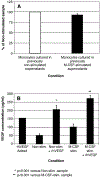
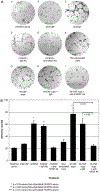
The impact of the immune response in malignancy is poorly understood. While immune cells can destroy transformed cells, the targeting and accumulation of monocytes and macrophages at tumor sites may promote tumor metastases. The growth factor M-CSF is important in promoting monocyte survival. Since M-CSF(-/-) mice are protected against tumor metastases, we hypothesized that M-CSF induced monocytes to produce angiogenic factors that facilitate metastases. In this study we demonstrate that recombinant human M-CSF induces freshly isolated normal human monocytes to produce and release the growth factor vascular endothelial growth factor (VEGF) in a dose-dependent manner, which peaked at 5 days in culture. VEGF released by these monocytes is biologically active, as cell-free supernatants from these M-CSF-stimulated monocytes induced tube formation in HUVEC. Network formation by these HUVECs after treatment with supernatants from monocytes stimulated with M-CSF were inhibited by anti-VEGF, but not by the isogenic control, Abs. Collectively, these data support an important role for M-CSF and monocytes in VEGF production and angiogenesis.
Figures
References
-
- Dvorak HF 2000. VPF/VEGF and the angiogenic response. Semin. Perinatol 24:75. - PubMed
-
- Hamada K, Oike Y, Takakura N, Ito Y, Jussila L, Dumont DJ, Alitalo K, and Suda T. 2000. VEGF-C signaling pathways through VEGFR-2 and VEGFR-3 in vasculoangiogenesis and hematopoiesis. Blood 96:3793. - PubMed
-
- Romano DP, Mangoni A, Zambruno G, Spinetti G, Melillo G, Napolitano M, and Capogrossi MC. 2002. Adenovirus-mediated VEGF(165) gene transfer enhances wound healing by promoting angiogenesis in CD1 diabetic mice. Gene Ther. 9:1271. - PubMed
-
- Senger DR, Van De WL, Brown LF, Nagy JA, Yeo KT, Yeo TK, Berse B, Jackman RW, Dvorak AM, and Dvorak HF. 1993. Vascular permeability factor (VPF, VEGF) in tumor biology. Cancer Metastasis Rev. 12:303. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

