An ultrasensitive Ca2+/calmodulin-dependent protein kinase II-protein phosphatase 1 switch facilitates specificity in postsynaptic calcium signaling
- PMID: 12928489
- PMCID: PMC193592
- DOI: 10.1073/pnas.1932759100
An ultrasensitive Ca2+/calmodulin-dependent protein kinase II-protein phosphatase 1 switch facilitates specificity in postsynaptic calcium signaling
Abstract
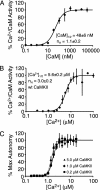
The strength of hippocampal synapses can be persistently increased by signals that activate Ca2+/calmodulin-dependent protein kinase II (CaMKII). This CaMKII-dependent long-term potentiation is important for hippocampal learning and memory. In this work we show that CaMKII exhibits an intriguing switch-like activation that likely is important for changes in synaptic strength. We found that autophosphorylation of CaMKII by itself showed a steep dependence on Ca2+ concentration [Hill coefficient (nH) approximately 5]. However, an even steeper Ca2+ dependence (nH approximately 8) was observed when autophosphorylation is balanced by the dephosphorylation activity of protein phosphatase 1 (PP1). This autophosphorylation-dephosphorylation switch was found to be reversible because PP1 dephosphorylates CaMKII when Ca2+ is lowered to a basal level. The switch-like response of a CaMKII-PP1 system suggests that CaMKII and PP1 may function together as a simple molecular device that specifically translates only strong Ca2+ signals into all-or-none potentiation of individual hippocampal synapses.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

