In vitro co-stimulation with anti-CD28 synergizes with IL-12 in the generation of T cell immune responses to leukaemic cells; a strategy for ex-vivo generation of CTL for immunotherapy
- PMID: 12930376
- PMCID: PMC1808782
- DOI: 10.1046/j.1365-2249.2003.02235.x
In vitro co-stimulation with anti-CD28 synergizes with IL-12 in the generation of T cell immune responses to leukaemic cells; a strategy for ex-vivo generation of CTL for immunotherapy
Abstract
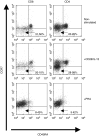
The existence of an immune based graft-versus-leukaemia (GvL) effect highlighted the prospect of managing relapsed leukaemias with T cell-based adoptive immunotherapy. Thus, various strategies have been explored for the in vitro expansion of acute myeloid leukaemia (AML)-specific T cells. In a popular approach, AML blasts have been genetically modified to express co-stimulatory molecules essential for effective T cell priming. One such tactic has been the modification of AML cells to express the B7/CD80 co-stimulatory molecule that binds to CD28 on T cells initiating events that culminate in enhanced cytokine production, proliferation and development of effector functions by T cells. The success of these strategies has been limited by difficulties in attaining sufficient transduction efficiencies and associated high levels of CD80 expression. We demonstrate that these problems can be circumvented by using anti-CD28 monoclonal antibody. Furthermore, we show that the synergistic relationship between CD80/CD28 pathway and interleukin 12 cytokine (IL-12), documented in the generation of cytotoxic T lymphocytes (CTL) for solid tumours, also applies to AML. CD28/IL-12 synergy facilitated the proliferation of allogeneic T cells in response to stimulation with primary AML blasts. The synergy also favoured generation of a Th1-type immune response, evidenced by gamma interferon (IFN-gamma) secretion and facilitated naive and memory T cell proliferation. Unlike some methods of in vitro T cell expansion, use of CD28/IL-12 synergy left T cells in the physiologically appropriate CD45RA-/CCR7- subsets known to be associated with immediate cytotoxic functions.
Figures
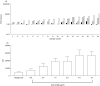
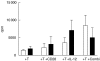
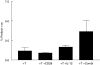
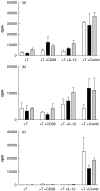
 , IL-12 cytokine (▪) or anti-CD28 in combination with IL-12 cytokine (□). Fold increase was calculated relative to the proliferation of T cells stimulated with AML blasts alone. (b) The effect of anti-CD28 on allogeneic T cell response to AML blasts plus IL-12.
, IL-12 cytokine (▪) or anti-CD28 in combination with IL-12 cytokine (□). Fold increase was calculated relative to the proliferation of T cells stimulated with AML blasts alone. (b) The effect of anti-CD28 on allogeneic T cell response to AML blasts plus IL-12.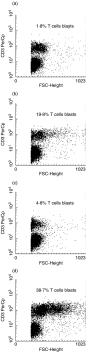

Similar articles
-
Restimulation of tumour-specific immunity in a patient with AML following injection with B7-1 positive autologous blasts.Leuk Res. 2003 Nov;27(11):1051-61. doi: 10.1016/s0145-2126(03)00058-4. Leuk Res. 2003. PMID: 12859998
-
Differential graft-versus-leukaemia effect by CD28 and CD40 co-stimulatory blockade after graft-versus-host disease prophylaxis.Clin Exp Immunol. 2002 Jul;129(1):61-8. doi: 10.1046/j.1365-2249.2002.01857.x. Clin Exp Immunol. 2002. PMID: 12100023 Free PMC article.
-
Modulation of experimental blood stage malaria through blockade of the B7/CD28 T-cell costimulatory pathway.Immunology. 1999 Mar;96(3):498-504. doi: 10.1046/j.1365-2567.1999.00718.x. Immunology. 1999. PMID: 10233733 Free PMC article.
-
Th2 and Tc2 cells in the regulation of GVHD, GVL, and graft rejection: considerations for the allogeneic transplantation therapy of leukemia and lymphoma.Leuk Lymphoma. 2000 Jul;38(3-4):221-34. doi: 10.3109/10428190009087014. Leuk Lymphoma. 2000. PMID: 10830730 Review.
-
T cell co-stimulation and in vivo tolerance.Curr Opin Immunol. 1993 Oct;5(5):747-52. doi: 10.1016/0952-7915(93)90132-c. Curr Opin Immunol. 1993. PMID: 7694594 Review.
Cited by
-
Lytic activity against primary AML cells is stimulated in vitro by an autologous whole cell vaccine expressing IL-2 and CD80.Cancer Immunol Immunother. 2010 Mar;59(3):379-88. doi: 10.1007/s00262-009-0756-x. Epub 2009 Aug 27. Cancer Immunol Immunother. 2010. PMID: 19711075 Free PMC article.
-
The cytokine network in acute myeloid leukemia.Front Immunol. 2022 Sep 28;13:1000996. doi: 10.3389/fimmu.2022.1000996. eCollection 2022. Front Immunol. 2022. PMID: 36248849 Free PMC article. Review.
-
The characteristics of circRNA as competing endogenous RNA in pathogenesis of acute myeloid leukemia.BMC Cancer. 2021 Mar 15;21(1):277. doi: 10.1186/s12885-021-08029-7. BMC Cancer. 2021. PMID: 33722210 Free PMC article.
References
-
- Boyer MW, Vallera DA, Taylor PA, et al. The role of B7 co-stimulation by murine acute myeloid leukaemia in the generation and function of CD8+ T-cell line with potent in vivo graft-versus-leukaemia properties. Blood. 1997;89:3477–85. - PubMed
-
- Mutis T, Verdijk R, Schrama E, Esendam B, Brand A, Goulmy E. Feasibility of immunotherapy of relasped leukaemia with ex vivo – generated cytotoxic T lymphocytes specific for hematopoietic system-restricted minor histocompatibility antigens. Blood. 1999;93:2336–41. - PubMed
-
- Notter M, Willinger T, Erben U, Thiel E. Targeting of a B7-1 (CD80) immunoglobulin G fusion protein to acute myeloid leukemia blasts increases their co-stimulatory activity for autologous remission T cells. Blood. 2001;97:3138–45. - PubMed
-
- Lenschow DJ, Walunas TL, Bluestone JA. CD28/B7 system of T cell co-stimulation. Annu Rev Immunol. 1996;14:233. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

