Chlamydomonas fla mutants reveal a link between deflagellation and intraflagellar transport
- PMID: 12930563
- PMCID: PMC194436
- DOI: 10.1186/1471-2121-4-11
Chlamydomonas fla mutants reveal a link between deflagellation and intraflagellar transport
Abstract
Background: Cilia and flagella are often lost in anticipation of mitosis or in response to stress. There are two ways that a cell can lose its flagella: resorption or deflagellation. Deflagellation involves active severing of the axoneme at the base of the flagellum; this process is defective in Chlamydomonas fa mutants. In contrast, resorption has been thought to occur as a consequence of constitutive disassembly at the tip in the absence of continued assembly, which requires intraflagellar transport (IFT). Chlamydomonas fla mutants are unable to build and maintain flagella due to defects in IFT.
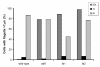
Results: fla10 cells, which are defective in kinesin-II, the anterograde IFT motor, resorb their flagella at the restrictive temperature (33 degrees C), as previously reported. We find that in standard media containing approximately 300 microM calcium, fla10 cells lose flagella by deflagellation at 33 degrees C. This temperature-induced deflagellation of a fla mutant is not predicted by the IFT-based model for flagellar length control. Other fla mutants behave similarly, losing their flagella by deflagellation instead of resorption, if adequate calcium is available. These data suggest a new model whereby flagellar resorption involves active disassembly at the base of the flagellum via a mechanism with components in common with the severing machinery of deflagellation. As predicted by this model, we discovered that deflagellation stimuli induce resorption if deflagellation is blocked either by mutation in a FA gene or by lack of calcium. Further support for this model comes from our discovery that fla10-fa double mutants resorb their flagella more slowly than fla10 mutants.
Conclusions: Deflagellation of the fla10 mutant at the restrictive temperature is indicative of an active disassembly signal, which can manifest as either resorption or deflagellation. We propose that when IFT is halted by either an inactivating mutation or a cellular signal, active flagellar disassembly is initiated. This active disassembly is distinct from the constitutive disassembly which plays a role in flagellar length control.
Figures
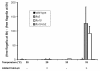
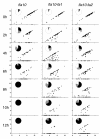
 wild-type,
wild-type,  fla2,
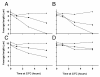
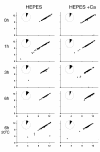
fla2,  fla10. (A) Incubation in HEPES, including contribution of bald and uniflagellate cells.(B) Incubation in HEPES+Ca, including contribution of bald and uniflagellate cells.(C) The same samples as (A), but averages exclude contributions of zero-length flagella.(D) The same samples as (B), but averages exclude contribution of zero-length flagella.
fla10. (A) Incubation in HEPES, including contribution of bald and uniflagellate cells.(B) Incubation in HEPES+Ca, including contribution of bald and uniflagellate cells.(C) The same samples as (A), but averages exclude contributions of zero-length flagella.(D) The same samples as (B), but averages exclude contribution of zero-length flagella.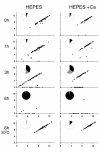
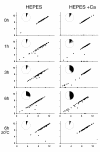

References
-
- Cole DG, Diener DR, Himelblau AL, Beech PL, Fuster JC, Rosenbaum JL. Chlamydomonas kinesin-II-dependent intraflagellar transport (IFT): IFT particles contain proteins required for ciliary assembly in Caenorhabditis elegans sensory neurons. J Cell Biol. 1998;141:993–1008. doi: 10.1083/jcb.141.4.993. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

