Spike width reduction modifies the dynamics of short-term depression at a central synapse in the locust
- PMID: 12930784
- PMCID: PMC6740751
- DOI: 10.1523/JNEUROSCI.23-20-07461.2003
Spike width reduction modifies the dynamics of short-term depression at a central synapse in the locust
Abstract
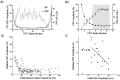
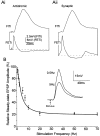
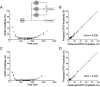
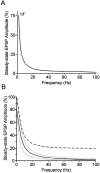
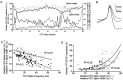
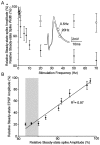
Short-term synaptic depression is an important component of computation within neural networks, but little is known of its contribution to information processing during synaptically generated spike trains. We analyzed short-term synaptic depression at a synapse between two identified motoneurons innervating the hind leg of the locust: the FETi-FlTi synapse (fast extensor tibiae-flexor tibiae). Brief electrical stimulation of a single hind leg proprioceptor, the lump receptor (LR), led to prolonged sequences of spikes in FETi, similar in number and frequency to those during natural kicking movements. Depression at the FETi-FlTi synapse during LR-evoked spike bursts was compared quantitatively to that during antidromic spike trains evoked by electrical stimulation of FETi in the extensor tibiae muscle, and by modeling. The magnitude of the short-term depression was significantly greater during LR-evoked spike trains. On the basis of the model parameters required to fit the depression, the FETi-FlTi synapse is predominantly used for transmitting the timing of the onset of FETi spiking rather than its spike rate. During LR-evoked spike trains, there was a rapid reduction in presynaptic spike width that did not occur during antidromic spike trains under physiological calcium concentrations. This produced a concomitant reduction in the amplitude of the FlTi EPSP, suggesting that it contributed to the differences between the two stimulation regimes. Differences in the short-term depression between synaptically evoked and antidromic spike trains emphasize that the properties of synaptic information transfer are dependent on the in vivo conditions at the synapse and may not be reproduced by in vitro spike trains.
Figures




References
-
- Abbott LF, Sen K, Varela JA, Nelson SB ( 1997) Synaptic depression and cortical gain control. Science 275: 220-222. - PubMed
-
- Burns MD, Usherwood PNR ( 1979) The control of walking in Orthoptera. II. Motor neurone activity in normal free-walking animals. J Exp Biol 79: 69-98.
-
- Burrows M ( 1995) Motor patterns during kicking movements in the locust. J Comp Physiol [A] 176: 289-305. - PubMed
-
- Burrows M ( 1996) The neurobiology of an insect brain, p 682. Oxford: Oxford University.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
