Apamin-sensitive small conductance calcium-activated potassium channels, through their selective coupling to voltage-gated calcium channels, are critical determinants of the precision, pace, and pattern of action potential generation in rat subthalamic nucleus neurons in vitro
- PMID: 12930791
- PMCID: PMC6740770
- DOI: 10.1523/JNEUROSCI.23-20-07525.2003
Apamin-sensitive small conductance calcium-activated potassium channels, through their selective coupling to voltage-gated calcium channels, are critical determinants of the precision, pace, and pattern of action potential generation in rat subthalamic nucleus neurons in vitro
Erratum in
- J Neurosci. 2004 Feb 4;25(5):following 1254
Abstract
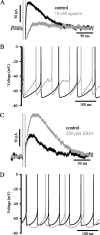
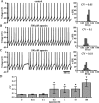
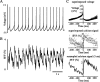
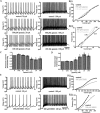
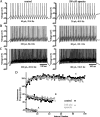
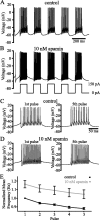
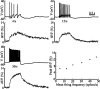
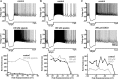
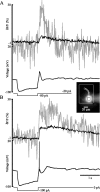
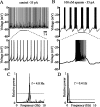
Distinct activity patterns in subthalamic nucleus (STN) neurons are observed during normal voluntary movement and abnormal movement in Parkinson's disease (PD). To determine how such patterns of activity are regulated by small conductance potassium (SK)/calcium-activated potassium (KCa) channels and voltage-gated calcium (Cav) channels, STN neurons were recorded in the perforated patch configuration in slices, [which were prepared from postnatal day 16 (P16)-P30 rats and held at 37 degrees C] and then treated with the SK KCa channel antagonist apamin or the SK KCa channel agonist 1-ethyl-2-benzimidazolinone or the Cav channel antagonists w-omega-conotoxin GVIA (Cav2.2-selective) or nifedipine (Cav1.2-1.3-selective) [corrected]. In other experiments, fura-2 was introduced as an indicator of intracellular calcium dynamics. A component of the current underlying single-spike afterhyperpolarization was sensitive to apamin, phase-locked to calcium entry via Cav2.2 channels, and necessary for precise, autonomous, single-spike oscillation. SK KCa/Cav2.2 channel coupling did not underlie spike-frequency adaptation but limited activity in response to current injection by encoding the accumulation of intracellular calcium, maintained the characteristic sigmoidal frequency-intensity relationship and generated a post-train afterhyperpolarization. In addition, SK KCa channels terminated rebound burst activity more effectively in neurons with short-duration bursts (<100 msec) than neurons with long-duration bursts (>100 msec), presumably through their activation by Cav3 channels. Cav1.2-1.3 channels were not strongly coupled to SK KCa channels and therefore supported secondary range and long-duration rebound burst firing. In summary, SK KCa channels play a fundamental role in autonomous, driven, and rebound activity and oppose the transition from autonomous, rhythmic, single-spike activity to burst firing in STN neurons.
Figures



Similar articles
-
Accumulation of cytoplasmic calcium, but not apamin-sensitive afterhyperpolarization current, during high frequency firing in rat subthalamic nucleus cells.J Physiol. 2008 Feb 1;586(3):817-33. doi: 10.1113/jphysiol.2007.141929. Epub 2007 Dec 6. J Physiol. 2008. PMID: 18063664 Free PMC article.
-
Selective coupling of T-type calcium channels to SK potassium channels prevents intrinsic bursting in dopaminergic midbrain neurons.J Neurosci. 2002 May 1;22(9):3404-13. doi: 10.1523/JNEUROSCI.22-09-03404.2002. J Neurosci. 2002. PMID: 11978817 Free PMC article.
-
D2-like dopamine receptors modulate SKCa channel function in subthalamic nucleus neurons through inhibition of Cav2.2 channels.J Neurophysiol. 2008 Feb;99(2):442-59. doi: 10.1152/jn.00998.2007. Epub 2007 Dec 19. J Neurophysiol. 2008. PMID: 18094105
-
Small-conductance calcium-activated potassium channels.Ann N Y Acad Sci. 1999 Apr 30;868:370-8. doi: 10.1111/j.1749-6632.1999.tb11298.x. Ann N Y Acad Sci. 1999. PMID: 10414306 Review.
-
KCa 2.2 (KCNN2): A physiologically and therapeutically important potassium channel.J Neurosci Res. 2023 Nov;101(11):1699-1710. doi: 10.1002/jnr.25233. Epub 2023 Jul 19. J Neurosci Res. 2023. PMID: 37466411 Free PMC article. Review.
Cited by
-
Silent plateau potentials, rhythmic bursts, and pacemaker firing: three patterns of activity that coexist in quadristable subthalamic neurons.Proc Natl Acad Sci U S A. 2006 Jan 3;103(1):183-8. doi: 10.1073/pnas.0506781102. Epub 2005 Dec 22. Proc Natl Acad Sci U S A. 2006. PMID: 16373507 Free PMC article.
-
Molecular and functional profiling of cell diversity and identity in the lateral superior olive, an auditory brainstem center with ascending and descending projections.Front Cell Neurosci. 2024 May 23;18:1354520. doi: 10.3389/fncel.2024.1354520. eCollection 2024. Front Cell Neurosci. 2024. PMID: 38846638 Free PMC article.
-
Computational model for control of hand movement in Parkinson's disease using deep brain stimulation.Exp Brain Res. 2025 Feb 23;243(3):74. doi: 10.1007/s00221-025-07026-7. Exp Brain Res. 2025. PMID: 39987542
-
Signal enhancement in the output stage of the basal ganglia by synaptic short-term plasticity in the direct, indirect, and hyperdirect pathways.Front Comput Neurosci. 2013 Jun 19;7:76. doi: 10.3389/fncom.2013.00076. eCollection 2013. Front Comput Neurosci. 2013. PMID: 23801960 Free PMC article.
-
Enhancement of excitatory synaptic integration by GABAergic inhibition in the subthalamic nucleus.J Neurosci. 2005 Sep 14;25(37):8505-17. doi: 10.1523/JNEUROSCI.1163-05.2005. J Neurosci. 2005. PMID: 16162932 Free PMC article.
References
-
- Abe Y, Furukawa K, Itoyama Y, Akaike N ( 1994) Glycine response in acutely dissociated ventromedial hypothalamic neuron of the rat: new approach with gramicidin perforated patch-clamp technique. J Neurophysiol 72: 1530-1537. - PubMed
-
- Aizenman CD, Linden DJ ( 1999) Regulation of the rebound depolarization and spontaneous firing patterns of deep nuclear neurons in slices of rat cerebellum. J Neurophysiol 82: 1697-1709. - PubMed
-
- Alexander JC, Barnwell LF, Schrader LA, Pfaffinger PJ, Sweatt JD, Anderson AE ( 2002) PKA phosphorylates the small conductance K+ channel subunit, SK2. Soc Neurosci Abstr 22: 217.6.
-
- Barry PH ( 1994) JPCalc: a software package for calculating liquid junction potential corrections in patch-clamp, intracellular, epithelial and bilayer measurements and for correcting junction potential measurements. J Neurosci Methods 51: 107-116. - PubMed
-
- Baufreton J, Garret M, Dovero S, Dufy B, Bioulac B, Taupignon A ( 2001) Activation of GABA(A) receptors in subthalamic neurons in vitro: properties of native receptors and inhibition mechanisms. J Neurophysiol 86: 75-85. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
