Circadian clock-controlled regulation of cGMP-protein kinase G in the nocturnal domain
- PMID: 12930792
- PMCID: PMC6740760
- DOI: 10.1523/JNEUROSCI.23-20-07543.2003
Circadian clock-controlled regulation of cGMP-protein kinase G in the nocturnal domain
Abstract
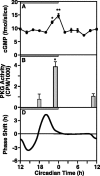
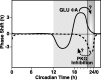
The suprachiasmatic nucleus (SCN) circadian clock exhibits a recurrent series of dynamic cellular states, characterized by the ability of exogenous signals to activate defined kinases that alter clock time. To explore potential relationships between kinase activation by exogenous signals and endogenous control mechanisms, we examined clock-controlled protein kinase G (PKG) regulation in the mammalian SCN. Signaling via the cGMP-PKG pathway is required for light- or glutamate (GLU)-induced phase advance in late night. Spontaneous cGMP-PKG activation occurred at the end of subjective night in free-running SCN in vitro. Phasing of the SCN rhythm in vitro was delayed by approximately 3 hr after treatment with guanylyl cyclase (GC) inhibitors, PKG inhibition, or antisense oligodeoxynucleotide (alphaODN) specific for PKG, but not PKA inhibitor or mismatched ODN. This sensitivity to GC-PKG inhibition was limited to the same 2 hr time window demarcated by clock-controlled activation of cGMP-PKG. Inhibition of the cGMP-PKG pathway at this time caused delays in the phasing of four endogenous rhythms: wheel-running activity, neuronal activity, cGMP, and Per1. Timing of the cGMP-PKG-necessary window in both rat and mouse depended on clock phase, established by the antecedent light/dark cycle rather than solar time. Because behavioral, neurophysiological, biochemical, and molecular rhythms showed the same temporal sensitivities and qualitative responses, we predict that clock-regulated GC-cGMP-PKG activation may provide a necessary cue as to clock state at the end of the nocturnal domain. Because sensitivity to phase advance by light-GLU-activated GC-cGMP-PKG occurs in juxtaposition, these signals may induce a premature shift to this PKG-necessary clock state.
Figures






Similar articles
-
Coupling of muscarinic cholinergic receptors and cGMP in nocturnal regulation of the suprachiasmatic circadian clock.J Neurosci. 1997 Jan 15;17(2):659-66. doi: 10.1523/JNEUROSCI.17-02-00659.1997. J Neurosci. 1997. PMID: 8987788 Free PMC article.
-
Rhythmicity of the cGMP-related signal transduction pathway in the mammalian circadian system.Am J Physiol Regul Integr Comp Physiol. 2001 May;280(5):R1348-55. doi: 10.1152/ajpregu.2001.280.5.R1348. Am J Physiol Regul Integr Comp Physiol. 2001. PMID: 11294753
-
cGMP-dependent protein kinase inhibitor blocks light-induced phase advances of circadian rhythms in vivo.Neurosci Lett. 1995 Sep 15;197(3):227-30. doi: 10.1016/0304-3940(95)11961-u. Neurosci Lett. 1995. PMID: 8552305
-
Signaling in the mammalian circadian clock: the NO/cGMP pathway.Neurochem Int. 2004 Nov;45(6):929-36. doi: 10.1016/j.neuint.2004.03.023. Neurochem Int. 2004. PMID: 15312987 Review.
-
Suprachiasmatic nucleus: the brain's circadian clock.Recent Prog Horm Res. 1999;54:33-58; discussion 58-9. Recent Prog Horm Res. 1999. PMID: 10548871 Review.
Cited by
-
Ryanodine receptors are regulated by the circadian clock and implicated in gating photic entrainment.J Neurosci. 2009 Sep 23;29(38):11717-9. doi: 10.1523/JNEUROSCI.3820-09.2009. J Neurosci. 2009. PMID: 19776257 Free PMC article. No abstract available.
-
A neuropeptide speeds circadian entrainment by reducing intercellular synchrony.Proc Natl Acad Sci U S A. 2013 Nov 12;110(46):E4355-61. doi: 10.1073/pnas.1307088110. Epub 2013 Oct 28. Proc Natl Acad Sci U S A. 2013. PMID: 24167276 Free PMC article.
-
Behavioral rhythmicity of mice lacking AhR and attenuation of light-induced phase shift by 2,3,7,8-tetrachlorodibenzo-p-dioxin.J Biol Rhythms. 2008 Jun;23(3):200-10. doi: 10.1177/0748730408316022. J Biol Rhythms. 2008. PMID: 18487412 Free PMC article.
-
A Symphony of Signals: Intercellular and Intracellular Signaling Mechanisms Underlying Circadian Timekeeping in Mice and Flies.Int J Mol Sci. 2019 May 13;20(9):2363. doi: 10.3390/ijms20092363. Int J Mol Sci. 2019. PMID: 31086044 Free PMC article. Review.
-
Redox and Antioxidant Modulation of Circadian Rhythms: Effects of Nitroxyl, N-Acetylcysteine and Glutathione.Molecules. 2021 Apr 26;26(9):2514. doi: 10.3390/molecules26092514. Molecules. 2021. PMID: 33925826 Free PMC article.
References
-
- Albrecht U, Sun ZS, Eichele G, Lee CC ( 1997) A differential response of two putative mammalian circadian regulators, mper1 and mper2, to light. Cell 91: 1055-1064. - PubMed
-
- Balsalobre A, Damiola F, Schibler U ( 1998) A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell 93: 929-937. - PubMed
-
- Comolli JC, Hastings JW ( 1999) Novel effects on the Gonyaulax circadian system produced by the protein kinase inhibitor staurosporine. J Biol Rhythms 14: 11-19. - PubMed
-
- Crosthwaite SK, Dunlap JC, Loros JJ ( 1997) Neurospora wc-1 and wc-2: transcription, photoresponses, and the origins of circadian rhythmicity. Science 276: 763-769. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
