Mechanisms of lateral inhibition in the olfactory bulb: efficiency and modulation of spike-evoked calcium influx into granule cells
- PMID: 12930793
- PMCID: PMC6740749
- DOI: 10.1523/JNEUROSCI.23-20-07551.2003
Mechanisms of lateral inhibition in the olfactory bulb: efficiency and modulation of spike-evoked calcium influx into granule cells
Abstract
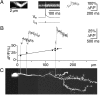
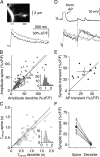
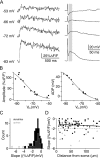
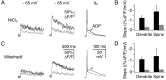
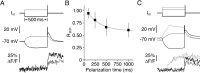
Granule cells are axonless local interneurons that mediate lateral inhibitory interactions between the principal neurons of the olfactory bulb via dendrodendritic reciprocal synapses. This unusual arrangement may give rise to functional properties different from conventional lateral inhibition. Although granule cells spike, little is known about the role of the action potential with respect to their synaptic output. To investigate the signals that underlie dendritic release in these cells, two-photon microscopy in rat brain slices was used to image calcium transients in granule cell dendrites and spines. Action potentials evoked calcium transients throughout the dendrites, with amplitudes increasing with distance from soma and attaining a plateau level within the external plexiform layer, the zone of granule cell synaptic output. Transient amplitudes were, on average, equal in size in spines and adjacent dendrites. Surprisingly, both spine and dendritic amplitudes were strongly dependent on membrane potential, decreasing with depolarization and increasing with hyperpolarization from rest. Both the current-voltage relationship and the time course of inactivation were consistent with the known properties of T-type calcium channels, and the voltage dependence was blocked by application of the T-type calcium channel antagonists Ni2+ and mibefradil. In addition, mibefradil reduced action potential-mediated synaptic transmission from granule to mitral cells. The implication of a transiently inactivating calcium channel in synaptic release from granule cells suggests novel mechanisms for the regulation of lateral inhibition in the olfactory bulb.
Figures

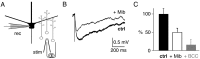
References
-
- Bezprozvanny I, Tsien RW ( 1995) Voltage-dependent blockade of diverse types of voltage-gated Ca2+ channels expressed in Xenopus oocytes by the Ca2+ channel antagonist mibefradil (Ro 40-5967). Mol Pharmacol 48: 540-549. - PubMed
-
- Buonviso N, Chaput MA, Berthommier F ( 1996) Similarity of granular-induced inhibitory periods in pairs of neighboring mitral/tufted cells. J Neurophysiol 76: 2393-2401. - PubMed
-
- Chaput MA ( 1986) Respiratory-phase-related coding of olfactory information in the olfactory bulb of awake freely breathing rabbits. Physiol Behav 36: 319-324. - PubMed
-
- Chen WR, Xiong W, Shepherd GM ( 2000) Analysis of relations between NMDA receptors and GABA release at olfactory bulb reciprocal synapses. Neuron 25: 625-633. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
