The meninges is a source of retinoic acid for the late-developing hindbrain
- PMID: 12930800
- PMCID: PMC6740759
- DOI: 10.1523/JNEUROSCI.23-20-07610.2003
The meninges is a source of retinoic acid for the late-developing hindbrain
Abstract
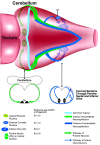
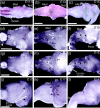
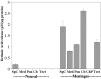
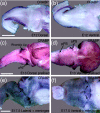
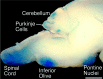
One general function for retinoic acid (RA) is pattern organization in the CNS. This regulatory factor has an essential role in spinal cord motor neuron and early posterior hindbrain development. In the anterior CNS, however, there is only a limited number of foci of RA synthesis, and less attention has been placed on regions such as the anterior hindbrain where RA synthesizing enzymes are absent. This study shows that a rich source of RA lies around the hindbrain from the RA synthetic enzyme retinaldehyde dehydrogenase-2 (RALDH2) present in the surrounding meninges and mesenchyme by embryonic day 13. RALDH2 is not distributed uniformly throughout the meninges but is restricted to territories over the developing hindbrain, suggesting that RA signaling may be localized to those regions. Further regulation of RA signaling is provided by the presence of a RA sink in the form of the CYP26B1 RA catabolic enzyme expressed in deeper regions of the brain. As a guide to the neural anatomy of hindbrain RA signaling, we used a mouse transgenic for a lacZ reporter gene driven by a RA response element (RAREhsplacZ) to identify regions of RA signaling. This reporter mouse provides evidence that RA signaling in the hindbrain after embryonic day 13 occurs in the regions of the cerebellum and precerebellar system adjacent to sources of RA, including the inferior olive and the pontine nuclei.
Figures




Similar articles
-
Novel retinoic acid generating activities in the neural tube and heart identified by conditional rescue of Raldh2 null mutant mice.Development. 2002 May;129(9):2271-82. doi: 10.1242/dev.129.9.2271. Development. 2002. PMID: 11959834 Free PMC article.
-
Beyond the neckless phenotype: influence of reduced retinoic acid signaling on motor neuron development in the zebrafish hindbrain.Dev Biol. 2004 Jul 1;271(1):119-29. doi: 10.1016/j.ydbio.2004.03.033. Dev Biol. 2004. PMID: 15196955
-
Independent roles for retinoic acid in segmentation and neuronal differentiation in the zebrafish hindbrain.Dev Biol. 2004 Jun 1;270(1):186-99. doi: 10.1016/j.ydbio.2004.02.022. Dev Biol. 2004. PMID: 15136149
-
ArRAnging the hindbrain.Trends Neurosci. 2002 Feb;25(2):61-4. doi: 10.1016/s0166-2236(02)02067-2. Trends Neurosci. 2002. PMID: 11814548 Review.
-
Heads or tails? Retinoic acid will decide.Bioessays. 1999 Oct;21(10):809-12. doi: 10.1002/(SICI)1521-1878(199910)21:10<809::AID-BIES2>3.0.CO;2-0. Bioessays. 1999. PMID: 10497330 Review.
Cited by
-
Isotretinoin and neuropsychiatric side effects: Continued vigilance is needed.J Affect Disord Rep. 2021 Dec;6:100230. doi: 10.1016/j.jadr.2021.100230. Epub 2021 Sep 10. J Affect Disord Rep. 2021. PMID: 37168254 Free PMC article.
-
Intrinsic retinoic acid synthesis is required for oligodendrocyte progenitor expansion during CNS remyelination.Front Cell Neurosci. 2025 Feb 24;19:1550139. doi: 10.3389/fncel.2025.1550139. eCollection 2025. Front Cell Neurosci. 2025. PMID: 40065911 Free PMC article.
-
Insights into the organization of dorsal spinal cord pathways from an evolutionarily conserved raldh2 intronic enhancer.Development. 2010 Feb;137(3):507-18. doi: 10.1242/dev.043257. Development. 2010. PMID: 20081195 Free PMC article.
-
The path from the choroid plexus to the subventricular zone: go with the flow!Front Cell Neurosci. 2012 Aug 9;6:34. doi: 10.3389/fncel.2012.00034. eCollection 2012. Front Cell Neurosci. 2012. PMID: 22907990 Free PMC article.
-
Retinoic acid-induced premature osteoblast-to-preosteocyte transitioning has multiple effects on calvarial development.Development. 2016 Apr 1;143(7):1205-16. doi: 10.1242/dev.129189. Epub 2016 Feb 22. Development. 2016. PMID: 26903503 Free PMC article.
References
-
- Abu-Abed S, MacLean G, Fraulob V, Chambon P, Petkovich M, Dolle P ( 2002) Differential expression of the retinoic acid-metabolizing enzymes CYP26A1 and CYP26B1 during murine organogenesis. Mech Dev 110: 173-177. - PubMed
-
- Abu-Abed S, Dolle P, Metzger D, Wood C, MacLean G, Chambon P, Petkovich M ( 2003) Developing with lethal RA levels: genetic ablation of RAR gamma can restore the viability of mice lacking Cyp26A1. Development 130: 1449-1459. - PubMed
-
- Afink GB, Nister M, Stassen BH, Joosten PH, Rademakers PJ, Bongcam-Rudloff E, Van Zoelen EJ, Mosselman S ( 1995) Molecular cloning and functional characterization of the human platelet-derived growth factor alpha receptor gene promoter. Oncogene 10: 1667-1672. - PubMed
-
- Altman J, Bayer SA ( 1987) Development of the precerebellar nuclei in the rat: I.-IV. J Comp Neurol 257: 477-552. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
