Developmental modulation of retinal wave dynamics: shedding light on the GABA saga
- PMID: 12930801
- PMCID: PMC6740765
- DOI: 10.1523/JNEUROSCI.23-20-07621.2003
Developmental modulation of retinal wave dynamics: shedding light on the GABA saga
Abstract
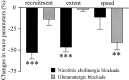
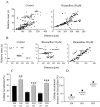
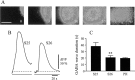
Embryonic spontaneous activity, in the form of propagating waves, is crucial for refining visual connections. To study what aspects of this correlated activity are instructive, we must first understand how their dynamics change with development and what factors trigger their disappearance after birth. Here we report that in the turtle retina, GABA, rather than glutamate and acetylcholine, influences developmental changes in wave dynamics. Using calcium imaging of the ganglion cell layer, we report how waves switch from fast and broad, when they emerge, to slow and narrow a few days before hatching, coinciding with the emergence of excitatory GABA(A) receptor-mediated activity. Around hatching, waves gradually become stationary patches, whereas GABA(A) shifts from excitatory to inhibitory, coinciding with the upregulation of the cotransporter KCC2, suggesting that changes in intracellular chloride underlie the shift. Dark-rearing from hatching causes correlated spontaneous activity to persist, whereas GABA(A) responses remain excitatory, and KCC2 expression is weaker. We conclude that GABA plays an important regulatory role during the maturation of retinal neural activity. Using a simple and elegant mechanism, namely the switch from excitatory to inhibitory, GABA(A) receptor-mediated activity is necessary and sufficient to cause retinal waves to stop propagating, ultimately leading to the disappearance of correlated spontaneous activity. Moreover, our results suggest that visual experience modulates the GABAergic switch.
Figures





Similar articles
-
GABA type-A activity controls its own developmental polarity switch in the maturing retina.J Neurosci. 2005 May 11;25(19):4801-5. doi: 10.1523/JNEUROSCI.0172-05.2005. J Neurosci. 2005. PMID: 15888655 Free PMC article.
-
The role of early neural activity in the maturation of turtle retinal function.J Anat. 2001 Oct;199(Pt 4):375-83. doi: 10.1046/j.1469-7580.2001.19940375.x. J Anat. 2001. PMID: 11693298 Free PMC article. Review.
-
A critical role of the strychnine-sensitive glycinergic system in spontaneous retinal waves of the developing rabbit.J Neurosci. 2001 Jul 15;21(14):5158-68. doi: 10.1523/JNEUROSCI.21-14-05158.2001. J Neurosci. 2001. PMID: 11438591 Free PMC article.
-
GABAergic control of retinal ganglion cell dendritic development.Neuroscience. 2012 Dec 27;227:30-43. doi: 10.1016/j.neuroscience.2012.09.040. Epub 2012 Sep 26. Neuroscience. 2012. PMID: 23022539
-
Retinal waves: mechanisms and function in visual system development.Cell Calcium. 2005 May;37(5):425-32. doi: 10.1016/j.ceca.2005.01.010. Cell Calcium. 2005. PMID: 15820390 Review.
Cited by
-
Dystrophin Is Required for the Proper Timing in Retinal Histogenesis: A Thorough Investigation on the mdx Mouse Model of Duchenne Muscular Dystrophy.Front Neurosci. 2020 Aug 31;14:760. doi: 10.3389/fnins.2020.00760. eCollection 2020. Front Neurosci. 2020. PMID: 32982660 Free PMC article.
-
Retinal wave behavior through activity-dependent refractory periods.PLoS Comput Biol. 2007 Nov;3(11):e245. doi: 10.1371/journal.pcbi.0030245. PLoS Comput Biol. 2007. PMID: 18052546 Free PMC article.
-
Age-dependent homeostatic plasticity of GABAergic signaling in developing retinal networks.J Neurosci. 2011 Aug 24;31(34):12159-64. doi: 10.1523/JNEUROSCI.3112-11.2011. J Neurosci. 2011. PMID: 21865458 Free PMC article.
-
Neural field models for latent state inference: Application to large-scale neuronal recordings.PLoS Comput Biol. 2019 Nov 4;15(11):e1007442. doi: 10.1371/journal.pcbi.1007442. eCollection 2019 Nov. PLoS Comput Biol. 2019. PMID: 31682604 Free PMC article.
-
Ocular Necessities: A Neuroethological Perspective on Vertebrate Visual Development.Brain Behav Evol. 2024;99(2):96-108. doi: 10.1159/000536035. Epub 2024 Mar 8. Brain Behav Evol. 2024. PMID: 38447544 Free PMC article. Review.
References
-
- Bansal A, Singer JH, Hwang BJ, Xu W, Beaudet A, Feller MB ( 2000) Mice lacking specific nicotinic acetylcholine receptor subunits exhibit dramatically altered spontaneous activity patterns and reveal a limited role for retinal waves in forming on and off circuits in the inner retina. J Neurosci 20: 7672-7681. - PMC - PubMed
-
- Ben-Ari Y ( 2002) Excitatory actions of GABA during development: the nature of the nurture. Nat Rev Neurosci 3: 728-739. - PubMed
-
- Cardinali DP, Golombek DA ( 1998) The rhythmic GABAergic system. Neurochem Res 23: 607-614. - PubMed
-
- Catsicas M, Bonness V, Becker D, Mobbs P ( 1998) Spontaneous Ca2+ transients and their transmission in the developing chick retina. Curr Biol 8: 283-286. - PubMed
-
- Crair MC ( 1999) Neuronal activity during development: permissive or instructive? Curr Opin Neurobiol 9: 88-93. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
