Developmental learning in a pain-related system: evidence for a cross-modality mechanism
- PMID: 12930812
- PMCID: PMC6740755
- DOI: 10.1523/JNEUROSCI.23-20-07719.2003
Developmental learning in a pain-related system: evidence for a cross-modality mechanism
Abstract
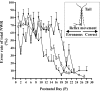
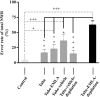
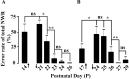
The nociceptive spinal reflex system performs highly precise sensorimotor transformations that require functionally specified synaptic strengths. The specification is gradually attained during early development and appears to be learning dependent. Here we determine the time course of this specification for heat-nociceptive tail withdrawal reflexes and analyze which types of primary afferents are important for the learning by applying various forms of noninvasive sensory deprivations. The percentage of erroneous heat-nociceptive tail withdrawal reflexes (i.e., movements directed toward the stimulation) decreased gradually from 64.1 +/- 2.5% (mean +/- SEM) to <10% during postnatal days 10-21. This improvement was completely blocked by anesthetizing the tail during the adaptation period, confirming that an experience-dependent mechanism is involved in the specification of synaptic strengths. However, the results show that the adaptation occurs to a significant extent despite local analgesia and protection of the tail from noxious input, provided that tactile sensitivity is preserved. Therefore, it appears that a nociceptive input is not necessary for the adaptation, and that input from tactile receptors can be used to guide the nociceptive synaptic organization during development. Sensory deprivation in the adult rat failed to affect the heat-nociceptive withdrawal reflex system, indicating that the adaptation has a "critical period" during early development. These findings provide a key to the puzzle of how pain-related systems can be functionally adapted through experience despite the rare occurrence of noxious input during early life.
Figures
Similar articles
-
Spontaneous movements: Effect of denervation and relation to the adaptation of nociceptive withdrawal reflexes in the rat.Physiol Behav. 2009 Dec 7;98(5):532-6. doi: 10.1016/j.physbeh.2009.08.009. Epub 2009 Aug 26. Physiol Behav. 2009. PMID: 19715712
-
Developmental tuning in a spinal nociceptive system: effects of neonatal spinalization.J Neurosci. 1999 Dec 1;19(23):10397-403. doi: 10.1523/JNEUROSCI.19-23-10397.1999. J Neurosci. 1999. PMID: 10575037 Free PMC article.
-
Functional organization of the nociceptive withdrawal reflexes. II. Changes of excitability and receptive fields after spinalization in the rat.Exp Brain Res. 1992;90(3):469-78. doi: 10.1007/BF00230929. Exp Brain Res. 1992. PMID: 1426107
-
Activity-dependent development of tactile and nociceptive spinal cord circuits.Ann N Y Acad Sci. 2013 Mar;1279:97-102. doi: 10.1111/nyas.12033. Ann N Y Acad Sci. 2013. PMID: 23531007 Review.
-
Action-based sensory encoding in spinal sensorimotor circuits.Brain Res Rev. 2008 Jan;57(1):111-7. doi: 10.1016/j.brainresrev.2007.08.007. Epub 2007 Sep 5. Brain Res Rev. 2008. PMID: 17920132 Review.
Cited by
-
Selective Targeting of Serotonin 5-HT1a and 5-HT3 Receptors Attenuates Acute and Long-Term Hypersensitivity Associated With Neonatal Procedural Pain.Front Pain Res (Lausanne). 2022 Apr 27;3:872587. doi: 10.3389/fpain.2022.872587. eCollection 2022. Front Pain Res (Lausanne). 2022. PMID: 35571143 Free PMC article.
-
The development of descending serotonergic modulation of the spinal nociceptive network: a life span perspective.Pediatr Res. 2022 May;91(6):1361-1369. doi: 10.1038/s41390-021-01638-9. Epub 2021 Jul 13. Pediatr Res. 2022. PMID: 34257402 Review.
-
A postnatal switch in GABAergic control of spinal cutaneous reflexes.Eur J Neurosci. 2006 Jan;23(1):112-8. doi: 10.1111/j.1460-9568.2005.04529.x. Eur J Neurosci. 2006. PMID: 16420421 Free PMC article.
-
Activity-dependent modulation of glutamatergic signaling in the developing rat dorsal horn by early tissue injury.J Neurophysiol. 2009 Oct;102(4):2208-19. doi: 10.1152/jn.00520.2009. Epub 2009 Aug 12. J Neurophysiol. 2009. PMID: 19675290 Free PMC article.
-
Long-Term Consequences of Neonatal Injury.Can J Psychiatry. 2015 Apr;60(4):176-80. doi: 10.1177/070674371506000404. Can J Psychiatry. 2015. PMID: 26174217 Free PMC article. Review.
References
-
- Altman J, Bayer SA ( 1984) The development of the rat spinal cord. Advances in anatomy embryology and cell biology, Vol 85. Berlin, Heidelberg, New York, Tokyo: Springer-Verlag. - PubMed
-
- Bizzi E, Tresch MC, Saltiel P, d'Avella A ( 2000) New perspectives on spinal motor systems. Nat Rev Neurosci 1: 101-108. - PubMed
-
- Blumberg MS, Lucas DE ( 1994) Dual mechanisms of twitching during sleep in neonatal rats. Behav Neurosci 108: 1196-1202. - PubMed
-
- Bromm B, Treede RD ( 1984) Nerve fibre discharges, cerebral potentials and sensations induced by CO2 laser stimulation. Hum Neurobiol 3: 33-40. - PubMed
-
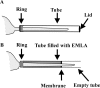
- Buckley MM, Benfield P ( 1993) Eutectic lidocaine/prilocaine cream. A review of the topical anaesthetic/analgesic efficacy of a eutectic mixture of local anaesthetics (EMLA). Drugs 46: 126-151. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
