Minocycline inhibits caspase-independent and -dependent mitochondrial cell death pathways in models of Huntington's disease
- PMID: 12930891
- PMCID: PMC193587
- DOI: 10.1073/pnas.1832501100
Minocycline inhibits caspase-independent and -dependent mitochondrial cell death pathways in models of Huntington's disease
Abstract
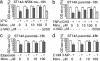
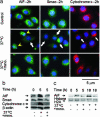
Minocycline is broadly protective in neurologic disease models featuring cell death and is being evaluated in clinical trials. We previously demonstrated that minocycline-mediated protection against caspase-dependent cell death related to its ability to prevent mitochondrial cytochrome c release. These results do not explain whether or how minocycline protects against caspase-independent cell death. Furthermore, there is no information on whether Smac/Diablo or apoptosis-inducing factor might play a role in chronic neurodegeneration. In a striatal cell model of Huntington's disease and in R6/2 mice, we demonstrate the association of cell death/disease progression with the recruitment of mitochondrial caspase-independent (apoptosis-inducing factor) and caspase-dependent (Smac/Diablo and cytochrome c) triggers. We show that minocycline is a drug that directly inhibits both caspase-independent and -dependent mitochondrial cell death pathways. Furthermore, this report demonstrates recruitment of Smac/Diablo and apoptosis-inducing factor in chronic neurodegeneration. Our results further delineate the mechanism by which minocycline mediates its remarkably broad neuroprotective effects.
Figures



References
-
- Chen, M., Ona, V. O., Li, M., Ferrante, R. J., Fink, K. B., Zhu, S., Bian, J., Guo, L., Farrell, L. A., Hersch, S. M., et al. (2000) Nat. Med. 6, 797-801. - PubMed
-
- Zhu, S., Stavrovskaya, I. G., Drozda, M., Kim, B. Y., Ona, V., Li, M., Sarang, S., Liu, A. S., Hartley, D. M., Wu, du C., et al. (2002) Nature 417, 74-78. - PubMed
-
- Sanchez Mejia, R. O., Ona, V. O., Li, M. & Friedlander, R. M. (2001). Neurosurgery 48, 1393-1399; discussion, 1399-1401. - PubMed
-
- Popovic, N., Schubart, A., Goetz, B. D., Zhang, S. C., Linington, C. & Duncan, I. D. (2002) Ann. Neurol. 51, 215-223. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

