Determination of DNA minor groove width in distamycin-DNA complexes by solid-state NMR
- PMID: 12930959
- PMCID: PMC212816
- DOI: 10.1093/nar/gkg720
Determination of DNA minor groove width in distamycin-DNA complexes by solid-state NMR
Abstract
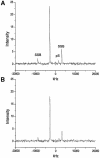
We have performed solid-state 31P-19F REDOR nuclear magnetic resonance (NMR) experiments to monitor changes in minor groove width of the oligonucleotide d(CGCAAA2'FUTGGC)*d(GCCAAT(pS)TT GCG) (A3T2) upon binding of the drug distamycin A at different stoichiometries. In the hydrated solid-state sample, the minor groove width for the unbound DNA, measured as the 2'FU7-pS19 inter-label distance, was 9.4 +/- 0.7 A, comparable to that found for similar A:T-rich DNAs. Binding of a single drug molecule is observed to cause a 2.4 A decrease in groove width. Subsequent addition of a second drug molecule results in a larger conformational change, expanding this minor groove width to 13.6 A, consistent with the results of a previous solution NMR study of the 2:1 complex. These 31P-19F REDOR results demonstrate the ability of solid-state NMR to measure distances of 7-14 A in DNA-drug complexes and provide the first example of a direct spectroscopic measurement of minor groove width in nucleic acids.
Figures



References
-
- Ketchem R.R., Hu,W. and Cross,T.A. (1993) High-resolution conformation of gramicidin A in a lipid bilayer by solid-state NMR. Science, 261, 1457–1460. - PubMed
-
- Opella S.J., Kim,Y. and Mcdonnell,P. (1994) Experimental nuclear magnetic resonance studies of membrane proteins. Methods Enzymol., 239, 536–560. - PubMed
-
- Studelska D.R., Klug,C.A., Beusen,D.D., McDowell,L.M. and Schaefer,J. (1996) Long-range distance measurements of protein binding sites by rotational-echo double resonance NMR. J. Am. Chem. Soc., 118, 5476–5477.
-
- Merritt M.E., Christensen,A.M., Kramer,K., Hopkins,T. and Schaefer,J. (1996) Detection of intercatechol cross-links in insect cuticle by solid-state carbon-13 and nitrogen-15 NMR. J. Am. Chem. Soc., 118, 11278–11282.
-
- Wang J., Balazs,Y.S. and Thompson,L.K. (1997) Solid-state REDOR NMR distance measurements at the ligand site of a bacterial chemotaxis membrane receptor. Biochemistry, 36, 1699–1703. - PubMed

