Dissecting interdomain communication within cAPK regulatory subunit type IIbeta using enhanced amide hydrogen/deuterium exchange mass spectrometry (DXMS)
- PMID: 12930997
- PMCID: PMC2323995
- DOI: 10.1110/ps.03166903
Dissecting interdomain communication within cAPK regulatory subunit type IIbeta using enhanced amide hydrogen/deuterium exchange mass spectrometry (DXMS)
Abstract
cAMP-dependent protein kinase (cAPK) is a heterotetramer containing a regulatory (R) subunit dimer bound to two catalytic (C) subunits and is involved in numerous cell signaling pathways. The C-subunit is activated allosterically when two cAMP molecules bind sequentially to the cAMP-binding domains, designated A and B (cAB-A and cAB-B, respectively). Each cAMP-binding domain contains a conserved Arg residue that is critical for high-affinity cAMP binding. Replacement of this Arg with Lys affects cAMP affinity, the structural integrity of the cAMP-binding domains, and cAPK activation. To better understand the local and long-range effects that the Arg-to-Lys mutation has on the dynamic properties of the R-subunit, the amide hydrogen/deuterium exchange in the RIIbeta subunit was probed by electrospray mass spectrometry. Mutant proteins containing the Arg-to-Lys substitution in either cAMP-binding domain were deuterated for various times and then, prior to mass spectrometry analysis, subjected to pepsin digestion to localize the deuterium incorporation. Mutation of this Arg in cAB-A (Arg230) causes an increase in amide hydrogen exchange throughout the mutated domain that is beyond the modest and localized effects of cAMP removal and is indicative of the importance of this Arg in domain organization. Mutation of Arg359 (cAB-B) leads to increased exchange in the adjacent cAB-A domain, particularly in the cAB-A domain C-helix that lies on top of the cAB-B domain and is believed to be functionally linked to the cAB-B domain. This interdomain communication appears to be a unidirectional pathway, as mutation of Arg230 in cAB-A does not effect dynamics of the cAB-B domain.
Figures




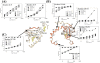


References
-
- Amieux, P.S., Howe, D.G., Knickerbocker, H., Lee, D.C., Su, T., Laslo, G.S., Idzerda, R.L., and McKnight, G.S. 2002. Increased basal PKA activity inhibits the formation of mesoderm-derived structures in the developing mouse embryo. J. Biol. Chem. 277 27294–27304. - PubMed
-
- Anand, G.S., Hughes, C.A., Jones, J.M., Taylor, S.S., and Komives, E.A. 2002. Amide H/2H exchange reveals communication between the cAMP and catalytic subunit-binding sites in the RIα subunit of protein kinase A. J. Mol. Biol. 323 377–386. - PubMed
-
- Andersen, M.D., Shaffer, J., Jennings, P.A., and Adams, J.A. 2001. Structural characterization of protein kinase A as a function of nucleotide binding: Hydrogen-deuterium exchange studies using matrix-assisted laser desorption ionization-time of flight mass spectrometry detection. J. Biol. Chem. 276 14204–14211. - PubMed
-
- Banky, P., Newlon, M.G., Roy, M., Morikis, D., Haste, N.M., Taylor, S.S., and Jennings, P.A. 2003. Unique dimeric surface topology mediates isoform-specific anchoring of PKA. J. Mol. Biol. (in press).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

