Gates and oscillators: a network model of the brain clock
- PMID: 12932086
- PMCID: PMC3271846
- DOI: 10.1177/0748730403253840
Gates and oscillators: a network model of the brain clock
Erratum in
- J Biol Rhythms. 2004 Jun;19(3):258
Abstract
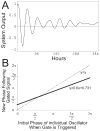
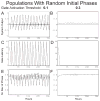
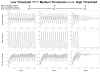
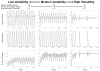
The suprachiasmatic nuclei (SCN) control circadian oscillations of physiology and behavior. Measurements of electrical activity and of gene expression indicate that these heterogeneous structures are composed of both rhythmic and nonrhythmic cells. A fundamental question with regard to the organization of the circadian system is how the SCN achieve a coherent output while their constituent independent cellular oscillators express a wide range of periods. Previously, the consensus output of individual oscillators had been attributed to coupling among cells. The authors propose a model that incorporates nonrhythmic "gate" cells and rhythmic oscillator cells with a wide range of periods, that neither requires nor excludes a role for interoscillator coupling. The gate provides daily input to oscillator cells and is in turn regulated (directly or indirectly) by the oscillator cells. In the authors' model, individual oscillators with initial random phases are able to self-assemble so as to maintain cohesive rhythmic output. In this view, SCN circuits are important for self-sustained oscillation, and their network properties distinguish these nuclei from other tissues that rhythmically express clock genes. The model explains how individual SCN cells oscillate independently and yet work together to produce a coherent rhythm.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

