Identification of human and mouse CatSper3 and CatSper4 genes: characterisation of a common interaction domain and evidence for expression in testis
- PMID: 12932298
- PMCID: PMC184451
- DOI: 10.1186/1477-7827-1-53
Identification of human and mouse CatSper3 and CatSper4 genes: characterisation of a common interaction domain and evidence for expression in testis
Abstract
Background: CatSper1 and CatSper2 are two recently identified channel-like proteins, which show sperm specific expression patterns. Through targeted mutagenesis in the mouse, CatSper1 has been shown to be required for fertility, sperm motility and for cAMP induced Ca2+ current in sperm. Both channels resemble a single pore forming repeat from a four repeat voltage dependent Ca2+ /Na+ channel. However, neither CatSper1 or CatSper2 have been shown to function as cation channels when transfected into cells, singly or in conjunction. As the pore forming units of voltage gated cation channels form a tetramer it has been suggested that the known CatSper proteins require additional subunits and/or interaction partners to function.
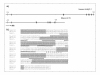
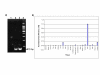
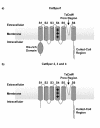
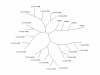
Results: Using in silico gene identification and prediction techniques, we have identified two further members of the CatSper family, CatSper3 and Catsper4. Each carries a single channel-forming domain with the predicted pore-loop containing the consensus sequence TxDxW. Each of the new CatSper genes has evidence for expression in the testis. Furthermore we identified coiled-coil protein-protein interaction domains in the C-terminal tails of each of the CatSper channels, implying that CatSper channels 1,2,3 and 4 may interact directly or indirectly to form a functional tetramer.
Conclusions: The topological and sequence relationship of CatSper1 and CatSper2 to the four repeat Ca2+ /Na+ channels suggested other members of this family may exist. We have identified a further two novel CatSper genes, conserved in both the human and mouse genomes. Furthermore, all four of the CatSper proteins are predicted to contain a common coiled-coil protein-protein interaction domain in their C-terminal tail. Coupled with expression data this leads to the hypothesis that the CatSper proteins form a functional hetero-tetrameric channel in sperm.
Figures


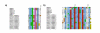

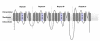
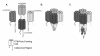
References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

