Keratocyte phenotype mediates proteoglycan structure: a role for fibroblasts in corneal fibrosis
- PMID: 12933807
- PMCID: PMC2877919
- DOI: 10.1074/jbc.M303292200
Keratocyte phenotype mediates proteoglycan structure: a role for fibroblasts in corneal fibrosis
Abstract
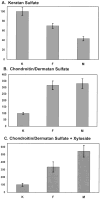
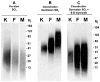
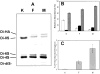
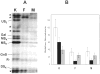
In pathological corneas, accumulation of fibrotic extracellular matrix is characterized by proteoglycans with altered glycosaminoglycans that contribute to the reduced transparency of scarred tissue. During wound healing, keratocytes in the corneal stroma transdifferentiate into fibroblasts and myofibroblasts. In this study, molecular markers were developed to identify keratocyte, fibroblast, and myofibroblast phenotypes in primary cultures of corneal stromal cells and the structure of glycosaminoglycans secreted by these cells was characterized. Quiescent primary keratocytes expressed abundant protein and mRNA for keratocan and aldehyde dehydrogenase class 3 and secreted proteoglycans containing macromolecular keratan sulfate. Expression of these marker compounds was reduced in fibroblasts and also in transforming growth factor-beta-induced myofibroblasts, which expressed high levels of alpha-smooth muscle actin, biglycan, and the extra domain A (EDA or EIIIA) form of cellular fibronectin. Collagen types I and III mRNAs were elevated in both fibroblasts and in myofibroblasts. Expression of these molecular markers clearly distinguishes the phenotypic states of stromal cells in vitro. Glycosaminoglycans secreted by fibroblasts and myofibroblasts were qualitatively similar to and differed from those of keratocytes. Chondroitin/dermatan sulfate abundance, chain length, and sulfation were increased as keratocytes became fibroblasts and myofibroblasts. Fluorophore-assisted carbohydrate electrophoresis analysis demonstrated increased N-acetylgalactosamine sulfation at both 4- and 6-carbons. Hyaluronan, absent in keratocytes, was secreted by fibroblasts and myofibroblasts. Keratan sulfate biosynthesis, chain length, and sulfation were significantly reduced in both fibroblasts and myofibroblasts. The qualitatively similar expression of glycosaminoglycans shared by fibroblasts and myofibroblasts suggests a role for fibroblasts in deposition of non-transparent fibrotic tissue in pathological corneas.
Figures


References
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

