Early induction of humoral and cellular immune responses during experimental Mycobacterium avium subsp. paratuberculosis infection of calves
- PMID: 12933856
- PMCID: PMC187349
- DOI: 10.1128/IAI.71.9.5130-5138.2003
Early induction of humoral and cellular immune responses during experimental Mycobacterium avium subsp. paratuberculosis infection of calves
Abstract
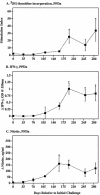
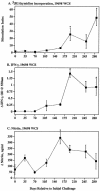
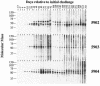
Johne's disease (paratuberculosis) of cattle is widespread and causes significant economic losses for producers due to decreased production and poor health of affected animals. The chronic nature of the disease and the lack of a reproducible model of infection hinder research efforts. In the present study, instillation of Mycobacterium avium subsp. paratuberculosis into the tonsillar crypts of neonatal calves resulted in peripheral colonization as detected by antemortem culture of feces and postmortem (320 days postchallenge) culture of intestinal tissues. Antigen-specific blastogenic, gamma interferon (IFN-gamma), and nitric oxide responses by blood mononuclear cells from infected calves exceeded prechallenge responses beginning 194 days postchallenge. Upon in vitro stimulation with paratuberculosis antigens, CD4(+) cells from infected calves proliferated, produced IFN-gamma, and increased expression of CD26 and CD45RO (indicative of an activated memory phenotype). Utilizing a lipoarabinomannan-based enzyme-linked immunosorbent assay, specific serum immunoglobulin was detected as early as 134 days postchallenge and generally increased after this time point. Two antigens of approximately 50 and approximately 60 kDa were particularly immunodominant early in infection, as shown by immunoblot with serum collected within 2 weeks postchallenge. Findings indicate that the intratonsillar inoculation route will prove useful as an experimental model for paratuberculosis infection. Additionally, this study confirms that mycobacteria-specific antibody is detectable early in the course of experimental Johne's disease, even preceding the development of specific cell-mediated responses.
Figures


Similar articles
-
Failure of antigen-stimulated gammadelta T cells and CD4+ T cells from sensitized cattle to upregulate nitric oxide and mycobactericidal activity of autologous Mycobacterium avium subsp. paratuberculosis-infected macrophages.Vet Immunol Immunopathol. 2007 Mar 15;116(1-2):1-12. doi: 10.1016/j.vetimm.2006.12.005. Epub 2007 Jan 9. Vet Immunol Immunopathol. 2007. PMID: 17275098 Free PMC article.
-
Early immune markers associated with Mycobacterium avium subsp. paratuberculosis infection in a neonatal calf model.Clin Vaccine Immunol. 2011 Mar;18(3):393-405. doi: 10.1128/CVI.00359-10. Epub 2011 Jan 12. Clin Vaccine Immunol. 2011. PMID: 21228140 Free PMC article.
-
Analysis of the immune response to Mycobacterium avium subsp. paratuberculosis in experimentally infected calves.Infect Immun. 2004 Dec;72(12):6870-83. doi: 10.1128/IAI.72.12.6870-6883.2004. Infect Immun. 2004. PMID: 15557608 Free PMC article.
-
Regulatory T cells in cattle and their potential role in bovine paratuberculosis.Comp Immunol Microbiol Infect Dis. 2012 May;35(3):233-9. doi: 10.1016/j.cimid.2012.01.004. Epub 2012 Jan 27. Comp Immunol Microbiol Infect Dis. 2012. PMID: 22285689 Review.
-
An update on Mycobacterium avium subspecies paratuberculosis antigens and their role in the diagnosis of Johne's disease.World J Microbiol Biotechnol. 2019 Jul 22;35(8):120. doi: 10.1007/s11274-019-2691-0. World J Microbiol Biotechnol. 2019. PMID: 31332578 Review.
Cited by
-
Evaluation of a Mycobacterium avium subsp. paratuberculosis leuD mutant as a vaccine candidate against challenge in a caprine model.Clin Vaccine Immunol. 2013 Apr;20(4):572-81. doi: 10.1128/CVI.00653-12. Epub 2013 Feb 13. Clin Vaccine Immunol. 2013. PMID: 23408524 Free PMC article.
-
Early antibody responses to experimental Mycobacterium bovis infection of cattle.Clin Vaccine Immunol. 2006 Jun;13(6):648-54. doi: 10.1128/CVI.00061-06. Clin Vaccine Immunol. 2006. PMID: 16760322 Free PMC article.
-
Members of the 30- to 32-kilodalton mycolyl transferase family (Ag85) from culture filtrate of Mycobacterium avium subsp. paratuberculosis are immunodominant Th1-type antigens recognized early upon infection in mice and cattle.Infect Immun. 2006 Jan;74(1):202-12. doi: 10.1128/IAI.74.1.202-212.2006. Infect Immun. 2006. PMID: 16368974 Free PMC article.
-
Bovine response to lipoarabinomannan vaccination and challenge with Mycobacterium paratuberculosis.Braz J Microbiol. 2013 Oct 30;44(2):511-4. doi: 10.1590/S1517-83822013000200029. eCollection 2013. Braz J Microbiol. 2013. PMID: 24294248 Free PMC article.
-
Vitamin d and leishmaniasis: Neither seasonal nor risk factor in canine host but potential adjuvant treatment through cbd103 expression.PLoS Negl Trop Dis. 2021 Aug 16;15(8):e0009681. doi: 10.1371/journal.pntd.0009681. eCollection 2021 Aug. PLoS Negl Trop Dis. 2021. PMID: 34398874 Free PMC article.
References
-
- Bannantine, J., and J. R. Stabel. 2000. HspX is present within Mycobacterium paratuberculosis-infected macrophages and is recognized by sera from some infected cattle. Vet. Microbiol. 76:343-358. - PubMed
-
- Beard, P. M., K. Stevenson, A. Pirie, K. Rudge, D. Buxton, S. M. Rhind, M. C. Sinclair, L. A. Wildblood, D. G. Jones, and J. M. Sharp. 2001. Experimental paratuberculosis in calves following inoculation with a rabbit isolate of Mycobacterium avium subsp. paratuberculosis. J. Clin. Microbiol. 39:3080-3084. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

