Protective mucosal Th2 immune response against Toxoplasma gondii by murine mesenteric lymph node dendritic cells
- PMID: 12933872
- PMCID: PMC187296
- DOI: 10.1128/IAI.71.9.5254-5265.2003
Protective mucosal Th2 immune response against Toxoplasma gondii by murine mesenteric lymph node dendritic cells
Abstract
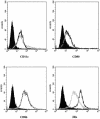
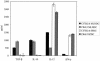
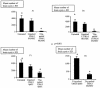
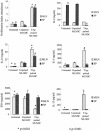
Toxoplasma gondii, an obligate intracellular parasite pathogen which initially invades the intestinal epithelium before disseminating throughout the body, may cause severe sequelae in fetuses and life-threatening neuropathy in immunocompromised patients. Immune protection is usually thought to be performed through a systemic Th1 response; considering the route of parasite entry it is important to study and characterize the local mucosal immune response to T. gondii. Despite considerable effort, Toxoplasma-targeted vaccines have proven to be elusive using conventional strategies. We report the use of mesenteric lymph node dendritic cells (MLNDCs) pulsed ex vivo with T. gondii antigens (TAg) as a novel investigation approach to vaccination against T. gondii-driven pathogenic processes. Using a murine model, we demonstrate in two genetically distinct mouse strains (C57BL/6 and CBA/J) that adoptively transferred TAg-pulsed MLNDCs elicit a mucosal Toxoplasma-specific Th2-biased immune response in vivo and confer strong protection against infection. We also observe that MLNDCs mostly traffic to the intestine where they enhance resistance by reduction in the mortality and in the number of brain cysts. Thus, ex vivo TAg-pulsed MLNDCs represent a powerful tool for the study of protective immunity to T. gondii, delivered through its natural route of entry. These findings might impact the design of vaccine strategies against other invasive microorganisms known to be delivered through digestive tract.
Figures



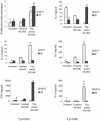
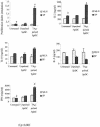
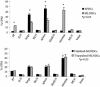
References
-
- Anjuere, F., P. Martin, I. Ferrero, M. L. Fraga, G. M. del Hoyo, N. Wright, and C. Ardavin. 1999. Definition of dendritic cell subpopulations present in the spleen, Peyer's patches, lymph nodes, and skin of the mouse. Blood 93:590-598. - PubMed
-
- Buzoni-Gatel, D., A. C. Lepage, I. H. Dimier-Poisson, D. T. Bout, and L. H. Kasper. 1997. Adoptive transfer of gut intraepithelial lymphocytes protects against murine infection with Toxoplasma gondii. J. Immunol. 158:5883-5889. - PubMed
-
- Chang, C.-C., A. Wright, and J. Punnonen. 2000. Monocyte-derived CD1a+ and CD1a- dendritic cell subsets differ in their cytokine production profiles, susceptibilities to transfection, and capacities to direct Th cell differentiation. J. Immunol. 165:3584-3591. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

