Calcineurin is essential for virulence in Candida albicans
- PMID: 12933882
- PMCID: PMC187310
- DOI: 10.1128/IAI.71.9.5344-5354.2003
Calcineurin is essential for virulence in Candida albicans
Abstract
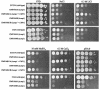
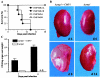
Calcineurin is a conserved Ca(2+)-calmodulin-activated, serine/threonine-specific protein phosphatase that regulates a variety of physiological processes, e.g., cell cycle progression, polarized growth, and adaptation to salt and alkaline pH stresses. In the pathogenic yeast Cryptococcus neoformans, calcineurin is also essential for growth at 37 degrees C and virulence. To investigate whether calcineurin plays a role in the virulence of Candida albicans, the major fungal pathogen of humans, we constructed C. albicans mutants in which both alleles of the CMP1 gene, encoding the calcineurin catalytic subunit, were deleted. The C. albicans Delta cmp1 mutants displayed hypersensitivity to elevated Na(+), Li(+), and Mn(2+) concentrations and to alkaline pH, phenotypes that have been described after calcineurin inactivation in the related yeast Saccharomyces cerevisiae. Unlike S. cerevisiae calcineurin mutants, which exhibit reduced susceptibility to high Ca(2+) concentrations, growth of C. albicans was inhibited in the presence of 300 mM CaCl(2) after the deletion of CMP1, demonstrating that there are also differences in calcineurin-mediated cellular responses between these two yeast species. In contrast to C. neoformans, inactivation of calcineurin did not cause temperature sensitivity in C. albicans. In addition, hyphal growth, an important virulence attribute of C. albicans, was not impaired in the Delta cmp1 mutants under a variety of inducing conditions. Nevertheless, the virulence of the mutants was strongly attenuated in a mouse model of systemic candidiasis, demonstrating that calcineurin signaling is essential for virulence in C. albicans.
Figures





References
-
- Bain, J. M., C. Stubberfield, and N. A. Gow. 2001. Ura-status-dependent adhesion of Candida albicans mutants. FEMS Microbiol Lett. 204:323-328. - PubMed
-
- Cole, M. F., W. H. Bowen, X. J. Zhao, and R. L. Cihlar. 1995. Avirulence of Candida albicans auxotrophic mutants in a rat model of oropharyngeal candidiasis. FEMS Microbiol Lett. 126:177-180. - PubMed
-
- Cruz, M. C., M. Del Poeta, P. Wang, R. Wenger, G. Zenke, V. F. Quesniaux, N. R. Movva, J. R. Perfect, M. E. Cardenas, and J. Heitman. 2000. Immunosuppressive and nonimmunosuppressive cyclosporine analogs are toxic to the opportunistic fungal pathogen Cryptococcus neoformans via cyclophilin-dependent inhibition of calcineurin. Antimicrob. Agents Chemother. 44:143-149. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

