Induction of T helper type 1 responses by a polysaccharide deacetylase from Cryptococcus neoformans
- PMID: 12933895
- PMCID: PMC187367
- DOI: 10.1128/IAI.71.9.5412-5417.2003
Induction of T helper type 1 responses by a polysaccharide deacetylase from Cryptococcus neoformans
Abstract
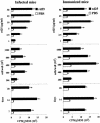
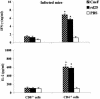
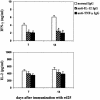
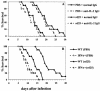
A 25-kDa cryptococcal deacetylase (d25) was found here to induce cell proliferation, as well as secretion of interleukin 2 and gamma interferon, but not interleukin 4, in spleen cells from d25-immunized or Cryptococcus neoformans-infected mice. The gamma interferon, but not the interleukin 2, response was required for the protective activities of d25 immunization in a murine cryptococcosis model.
Figures
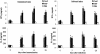
References
-
- Abrahams, J., and T. G. Gilleran. 1960. Studies on actively acquired resistance to experimental cryptococcosis in mice. J. Immunol. 85:629-635.
-
- Genovese, F., G. Mancuso, M. Cuzzola, C. Biondo, C. Beninati, D. Delfino, and G. Teti. 1999. Role of IL-10 in a neonatal mouse listeriosis model. J. Immunol. 163:2777-2782. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

