Pharmacokinetics, safety, and tolerability of oral posaconazole administered in single and multiple doses in healthy adults
- PMID: 12936975
- PMCID: PMC182636
- DOI: 10.1128/AAC.47.9.2788-2795.2003
Pharmacokinetics, safety, and tolerability of oral posaconazole administered in single and multiple doses in healthy adults
Abstract
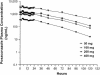
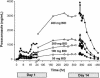
The pharmacokinetics, safety, and tolerability of posaconazole, an investigational triazole antifungal, were evaluated following the administration of rising single and multiple oral doses. A total of 103 healthy adults were enrolled in two phase I trials. Each study had a double-blind, placebo-controlled, parallel-group design with a rising single-dose (RSD) or rising multiple-dose (RMD) scheme. In the RSD study, subjects received single doses of posaconazole oral tablets (50 to 1200 mg) or placebo. In the RMD study, subjects received posaconazole oral tablets (50 to 400 mg) or placebo twice daily for 14 days. By using model-independent methods, the area under the plasma concentration-time curve and the maximum concentration in plasma were determined and used to assess dose proportionality. In the RSD study, the levels of posaconazole in plasma increased proportionally between the 50- and 800-mg dose range, with saturation of absorption occurring above 800 mg. Dose proportionality was also observed in the RMD study. In both studies, the apparent volume of distribution was large (range, 343 to 1341 liters) and the terminal-phase half-life was long (range, 25 to 31 h). Posaconazole was well tolerated at all dose levels, and the adverse events were not dose dependent. No clinically significant changes in clinical laboratory test values or electrocardiograms were observed. Following the administration of single and twice-daily rising doses, the level of posaconazole exposure increased in a dose-proportional manner. The long elimination-phase half-life of posaconazole supports once- or twice-daily dosing in clinical trials; however, additional studies are required to determine if further division of the dose will enhance exposure.
Figures



Similar articles
-
A new solid oral tablet formulation of posaconazole: a randomized clinical trial to investigate rising single- and multiple-dose pharmacokinetics and safety in healthy volunteers.J Antimicrob Chemother. 2012 Nov;67(11):2725-30. doi: 10.1093/jac/dks268. Epub 2012 Jul 24. J Antimicrob Chemother. 2012. PMID: 22833639 Free PMC article. Clinical Trial.
-
Pharmacokinetics and safety study of posaconazole intravenous solution administered peripherally to healthy subjects.Antimicrob Agents Chemother. 2015 Feb;59(2):1246-51. doi: 10.1128/AAC.04223-14. Epub 2014 Dec 15. Antimicrob Agents Chemother. 2015. PMID: 25512407 Free PMC article. Clinical Trial.
-
Pharmacokinetics and tolerability of intravenous posaconazole in healthy Chinese volunteers: a randomized, open-label and single-dose study.Pharmazie. 2020 Oct 1;75(10):491-493. doi: 10.1691/ph.2020.0512. Pharmazie. 2020. PMID: 33305723 Clinical Trial.
-
Posaconazole in immunocompromised pediatric patients.Expert Rev Anti Infect Ther. 2018 Jul;16(7):543-553. doi: 10.1080/14787210.2018.1490177. Epub 2018 Jul 3. Expert Rev Anti Infect Ther. 2018. PMID: 29912581 Review.
-
Posaconazole: an extended-spectrum triazole antifungal agent.Clin Ther. 2007 Sep;29(9):1862-86. doi: 10.1016/j.clinthera.2007.09.015. Clin Ther. 2007. PMID: 18035188 Review.
Cited by
-
Posaconazole exposure-response relationship: evaluating the utility of therapeutic drug monitoring.Antimicrob Agents Chemother. 2012 Jun;56(6):2806-13. doi: 10.1128/AAC.05900-11. Epub 2012 Mar 5. Antimicrob Agents Chemother. 2012. PMID: 22391534 Free PMC article. Review.
-
Therapeutic drug monitoring for triazoles: A needs assessment review and recommendations from a Canadian perspective.Can J Infect Dis Med Microbiol. 2014 Nov-Dec;25(6):327-43. doi: 10.1155/2014/340586. Can J Infect Dis Med Microbiol. 2014. PMID: 25587296 Free PMC article. Review.
-
Skin concentrations and pharmacokinetics of posaconazole after oral administration.Antimicrob Agents Chemother. 2010 May;54(5):1807-10. doi: 10.1128/AAC.01616-09. Epub 2010 Mar 1. Antimicrob Agents Chemother. 2010. PMID: 20194702 Free PMC article. Clinical Trial.
-
Pharmacokinetic/pharmacodynamic study of posaconazole delayed-release tablet in a patient with coexisting invasive aspergillosis and mucormycosis.Ther Clin Risk Manag. 2019 Apr 24;15:589-595. doi: 10.2147/TCRM.S203625. eCollection 2019. Ther Clin Risk Manag. 2019. PMID: 31114213 Free PMC article.
-
Development, clinical utility, and place in therapy of posaconazole for prevention and treatment of invasive fungal infections.Drug Des Devel Ther. 2010 Nov 4;4:299-311. doi: 10.2147/DDDT.S7773. Drug Des Devel Ther. 2010. PMID: 21116336 Free PMC article. Review.
References
-
- Barchiesi, F., A. M. Schimizzi, F. Caselli, D. Giannini, V. Camiletti, B. Fileni, A. Giacometti, L. F. Di Francesco, and G. Scalise. 2001. Activity of the new antifungal triazole, posaconazole, against Cryptococcus neoformans. J. Antimicrob. Chemother. 48:769-773. - PubMed
-
- Barone, J. A., B. L. Moskovitz, J. Guarnieri, A. E. Hassell, J. L. Colaizzi, R. H. Bierman, and L. Jessen. 1998. Food interaction and steady-state pharmacokinetics of itraconazole oral solution in healthy volunteers. Pharmacotherapy 18:295-301. - PubMed
-
- Cacciapuoti, A., D. Loebenberg, E. Corcoran, F. Menzel, Jr., E. L. Moss, Jr., C. Norris, M. Michalski, K. Raynor, J. Halpern, C. Mendrick, B. Arnold, B. Antonacci, R. Parmegiani, T. Yarosh-Tomaine, G. H. Miller, and R. S. Hare. 2000. In vitro and in vivo activities of SCH 56592 (posaconazole), a new triazole antifungal agent, against Aspergillus and Candida. Antimicrob. Agents Chemother. 44:2017-2022. - PMC - PubMed
-
- De Beule, K., and J. Van Gestel. 2001. Pharmacology of itraconazole. Drugs 61(Suppl. 1):27-37. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

