Okadaic-acid-induced inhibition of protein phosphatase 2A produces activation of mitogen-activated protein kinases ERK1/2, MEK1/2, and p70 S6, similar to that in Alzheimer's disease
- PMID: 12937126
- PMCID: PMC1868262
- DOI: 10.1016/S0002-9440(10)63445-1
Okadaic-acid-induced inhibition of protein phosphatase 2A produces activation of mitogen-activated protein kinases ERK1/2, MEK1/2, and p70 S6, similar to that in Alzheimer's disease
Abstract
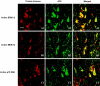
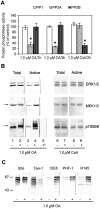
In Alzheimer's disease (AD) brain the activity of protein phosphatase (PP)-2A is compromised and that of the extracellular signal-regulated protein kinase (ERK1/2) of the mitogen-activated protein kinase (MAPK) family, which can phosphorylate tau, is up-regulated. We investigated whether a decrease in PP-2A activity could underlie the activation of these kinases and the abnormal hyperphosphorylation of tau. Rat brain slices, 400-microm-thick, kept under metabolically active conditions in oxygenated (95% O(2), 5% CO(2)) artificial CSF were treated with 1.0 micromol/L okadaic acid (OA) for 1 hour at 33 degrees C. Under this condition, PP-2A activity was decreased to approximately 35% of the vehicle-treated control slices, and activities of PP-1 and PP-2B were not affected. In the OA-treated slices, we observed a dramatic increase in the phosphorylation/activation of ERK1/2, MEK1/2, and p70 S6 kinase both immunohistochemically and by Western blots using phosphorylation-dependent antibodies against these kinases. Treatment of 6-microm sections of the OA-treated slices with purified PP-2A reversed the phosphorylation/activation of these kinases. Hyperphosphorylation of tau at several abnormal hyperphosphorylation sites was also observed, as seen in AD brain. These results suggest 1) that PP-2A down-regulates ERK1/2, MEK1/2, and p70 S6 kinase activities through dephosphorylation at the serine/threonine residues of these kinases, and 2) that in AD brain the decrease in PP-2A activity could have caused the activation of ERK1/2, MEK1/2, and p70 S6 kinase, and the abnormal hyperphosphorylation of tau both via an increase in its phosphorylation and a decrease in its dephosphorylation.
Figures







References
-
- Grundke-Iqbal I, Iqbal K, Quinlan M, Tung Y-C, Zaide MS, Wisniewski HM: Microtubule-associated protein tau, a component of Alzheimer-paired helical filaments. J Biol Chem 1986, 261:6084-6089 - PubMed
-
- Iqbal K, Grundke-Iqbal I, Zaidi T, Merz PA, Wen GY, Shaikh SS, Wisniewski HM, Alafuzoff I, Winblad B: Defective brain microtubule assembly in Alzheimer disease. Lancet 1986, 2:421-426 - PubMed
-
- Zhang Y, Dong Z, Nomura M, Zhong S, Chen N, Bode AM, Dong Z: Signal transduction pathways involved in phosphorylation and activation of p70S6K following exposure to UVA irradiation. J Biol Chem 2001, 276:20913-202923 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

