Complex genomic rearrangement of ALK loci associated with integrated human Epstein-Barr virus in a post-transplant myogenic liver tumor
- PMID: 12937132
- PMCID: PMC1868245
- DOI: 10.1016/S0002-9440(10)63451-7
Complex genomic rearrangement of ALK loci associated with integrated human Epstein-Barr virus in a post-transplant myogenic liver tumor
Abstract
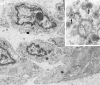
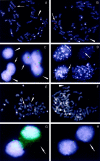
Epstein-Barr virus (EBV) is a ubiquitous viral agent, well known to be associated with lymphoid, epithelial, and smooth-muscle malignancies in immunocompromised individuals. This report describes a 10-year-old patient with an EBV-related liver tumor occurring after kidney transplantation. The neoplasm presented a phenotypic spectrum, ranging from a smooth-muscle tumor to an inflammatory pseudotumor (IPT). The neoplastic cells failed to disclose CD21, CD35, or ALK expression, the latter confirmed by reverse-transcription polymerase chain reaction. Cytogenetic analysis revealed a single clonal cell population showing 46,XY,del (2)(p23),der(3)t (2;3)(p23;q29),der(21) t(Y;21)(q12;p13) karyotype. By metaphase FISH analysis, the neoplastic cells demonstrated the presence of two molecularly different but related aberrant clones, one with the loss of one ALK allele and the second with translocation of the 3'end of ALK kinase domain on the der(3) chromosome. Using FISH with an EBV-specific and 3'end ALK DNA probes, a co-localization of the viral DNA and the ALK sequences was found on the der(3) chromosome. Metaphases with loss of rearranged ALK did not show integrated virus; instead, viral particles together with an associated 3'end ALK domain formed an ex-chromosomal, episomal-like type configuration. The interphase study, using dual-color 5'/3' end ALK FISH assay, revealed 30% of nuclei with only one fused signal, confirming the total loss of one ALK allele in the subset of tumor cells. A combined immunofluorescence and FISH study indicated this separate clonal variant to correspond to desmin-positive smooth-muscle cells. In contrast, desmin-negative myofibroblasts showed the presence of both normal and rearranged ALK alleles. Our results indicate that ALK locus may be a target of EBV integration, a hitherto unreported finding. Although the sustained clonal expansion in EBV-related smooth-muscle tumors/IPTs may depend on functions provided by the EBV oncogenic proteins, the tumor phenotype may be further modified by the secondary genomic rearrangements imposed by the virus during and/or after the integration event. In this respect, the observed phenotypic heterogeneity most likely reflects divergence during neoplastic progression, with the subsequent expansion of morphologically and molecularly distinct but cytogenetically related clones.
Figures
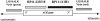



Similar articles
-
Absence of human herpesvirus-8 and Epstein-Barr virus in inflammatory myofibroblastic tumor with anaplastic large cell lymphoma kinase fusion gene.Pathol Int. 2006 Oct;56(10):584-90. doi: 10.1111/j.1440-1827.2006.02012.x. Pathol Int. 2006. PMID: 16984614
-
The association of Epstein-Barr virus with smooth-muscle tumors occurring after organ transplantation.N Engl J Med. 1995 Jan 5;332(1):19-25. doi: 10.1056/NEJM199501053320104. N Engl J Med. 1995. PMID: 7990861
-
Inflammatory pseudotumor of lymph node and spleen: an entity biologically distinct from inflammatory myofibroblastic tumor.Hum Pathol. 2001 Dec;32(12):1382-7. doi: 10.1053/hupa.2001.29679. Hum Pathol. 2001. PMID: 11774173
-
Epstein-Barr virus-associated smooth muscle tumour: a distinctive mesenchymal tumour of immunocompromised individuals.Pathology. 2002 Jun;34(3):245-9. doi: 10.1080/00313020220131309. Pathology. 2002. PMID: 12109785 Review.
-
Follicular dendritic cell tumor of the liver. Evidence for an Epstein-Barr virus-related clonal proliferation of follicular dendritic cells.Am J Surg Pathol. 1996 Mar;20(3):313-24. doi: 10.1097/00000478-199603000-00008. Am J Surg Pathol. 1996. PMID: 8772785 Review.
Cited by
-
Brain involvement in multicentric Epstein-Barr virus-associated smooth muscle tumours in a child after kidney transplantation.Virchows Arch. 2004 Apr;444(4):387-91. doi: 10.1007/s00428-004-0975-7. Virchows Arch. 2004. PMID: 15143769
-
Sarcomas other than Kaposi sarcoma occurring in immunodeficiency: interpretations from a systematic literature review.Curr Opin Oncol. 2012 Sep;24(5):537-46. doi: 10.1097/CCO.0b013e328355e115. Curr Opin Oncol. 2012. PMID: 22729152 Free PMC article.
-
A Clinicopathology Review and Update of Epstein-Barr Virus-Associated Mesenchymal Tumors.Cancers (Basel). 2023 Nov 24;15(23):5563. doi: 10.3390/cancers15235563. Cancers (Basel). 2023. PMID: 38067267 Free PMC article. Review.
References
-
- Lee ES, Locker J, Nalesnik M, Reyes J, Jaffe R, Alashari M, Nour B, Tzakis A, Dickman PS: The association of Epstein-Barr virus with smooth-muscle tumors occurring after organ transplantation. N Engl J Med 1995, 332:19-25 - PubMed
-
- Davidoff AM, Hebra A, Clark BJ, 3rd, Tomaszewski JE, Montone KT, Ruchelli E, Lan HT: Epstein-Barr virus-associated hepatic smooth-muscle neoplasm in a cardiac transplant recipient. Transplantation 1996, 61:515-517 - PubMed
-
- Wu TT, Swerdlow SH, Locker J, Bahler D, Randhawa P, Yunis EJ, Dickman PS, Nalesnik MA: Recurrent Epstein-Barr virus-associated lesions in organ transplant recipients. Hum Pathol 1996, 27:157-164 - PubMed
-
- Rogatsch H, Bonatti H, Menet A, Larcher C, Feichtinger H, Dirnhofer S: Epstein-Barr virus-associated multicentric leiomyosarcoma in an adult patient after heart transplantation: case report and review of the literature. Am J Surg Pathol 2000, 24:614-6215 - PubMed
-
- Cheuk W, Li PC, Chan JK: Epstein-Barr virus-associated smooth-muscle tumour: adistinctive mesenchymal tumour of immunocompromised individuals. Pathology 2002, 34:245-249 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

