Hypermethylation of the human glutathione S-transferase-pi gene (GSTP1) CpG island is present in a subset of proliferative inflammatory atrophy lesions but not in normal or hyperplastic epithelium of the prostate: a detailed study using laser-capture microdissection
- PMID: 12937133
- PMCID: PMC1868250
- DOI: 10.1016/s0002-9440(10)63452-9
Hypermethylation of the human glutathione S-transferase-pi gene (GSTP1) CpG island is present in a subset of proliferative inflammatory atrophy lesions but not in normal or hyperplastic epithelium of the prostate: a detailed study using laser-capture microdissection
Abstract
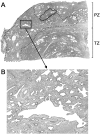
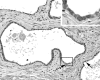
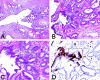
Somatic inactivation of the glutathione S-transferase-pi gene (GSTP1) via CpG island hypermethylation occurs early during prostate carcinogenesis, present in approximately 70% of high-grade prostatic intraepithelial neoplasia (high-grade PIN) lesions and more than 90% of adenocarcinomas. Recently, there has been a resurgence of the concept that foci of prostatic atrophy (referred to as proliferative inflammatory atrophy or PIA) may be precursor lesions for the development of prostate cancer and/or high-grade PIN. Many of the cells within PIA lesions contain elevated levels of GSTP1, glutathione S-transferase-alpha (GSTA1), and cyclooxygenase-II proteins, suggesting a stress response. Because not all PIA cells are positive for GSTP1 protein, we hypothesized that some of the cells within these regions acquire GSTP1 CpG island hypermethylation, increasing the chance of progression to high-grade PIN and/or adenocarcinoma. Separate regions (n =199) from 27 formalin-fixed paraffin-embedded prostates were microdissected by laser-capture microdissection (Arcturus PixCell II). These regions included normal epithelium (n = 48), hyperplasticepithelium from benign prostatic hyperplasia nodules (n = 22), PIA (n = 64), high-grade PIN (n = 32), and adenocarcinoma (n = 33). Genomic DNA was isolated and assessed for GSTP1 CpG island hypermethylation by methylation-specific polymerase chain reaction. GSTP1 CpG island hypermethylation was not detected in normal epithelium (0 of 48) or in hyperplastic epithelium (0 of 22), but was found in 4 of 64 (6.3%) PIA lesions. The difference in the frequency of GSTP1 CpG island hypermethylation between normal or hyperplastic epithelium and PIA was statistically significant (P = 0.049). Similar to studies using nonmicrodissected cases, hypermethylation was found in 22 of 32 (68.8%) high-grade PIN lesions and in 30 of 33 (90.9%) adenocarcinoma lesions. Unlike normal or hyperplastic epithelium, GSTP1 CpG island hypermethylation can be detected in some PIA lesions. These data support the hypothesis that atrophic epithelium in a subset of PIA lesions may lead to high-grade PIN and/or adenocarcinoma. Because these atrophic lesions are so prevalent and extensive, even though only a small subset contains this somatic DNA alteration, the clinical impact may be substantial.
Figures



References
-
- Putzi MJ, De Marzo AM: Prostate pathology: histologic and molecular perspectives. Hematol Oncol Clin North Am 2001, 15:407-421 - PubMed
-
- Ruska KM, Sauvageot J, Epstein JI: Histology and cellular kinetics of prostatic atrophy. Am J Surg Pathol 1998, 22:1073-1077 - PubMed
-
- McNeal JE: Normal histology of the prostate. Am J Surg Pathol 1988, 12:619-633 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

