Nitration of tau protein is linked to neurodegeneration in tauopathies
- PMID: 12937143
- PMCID: PMC1868254
- DOI: 10.1016/S0002-9440(10)63462-1
Nitration of tau protein is linked to neurodegeneration in tauopathies
Erratum in
- Am J Pathol. 2003 Dec;163(6):2645
Abstract
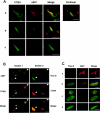
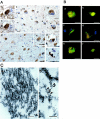
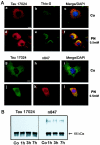
Oxidative and nitrative injury is implicated in the pathogenesis of Alzheimer's disease (AD) and Down syndrome (DS), but no direct evidence links this type of injury to the formation of neurofibrillary tau lesions. To address this, we generated a monoclonal antibody (mAb), n847, which recognizes nitrated tau and alpha-synuclein. n847 detected nitrated tau in the insoluble fraction of AD, corticobasal degeneration (CBD), and Pick's disease (PiD) brains by Western blots. Immunohistochemistry (IHC) showed that n847 labeled neurons in the hippocampus and neocortex of AD and DS brains. Double-label immunofluorescence with n847 and an anti-tau antibody revealed partial co-localization of tau and n847 positive tangles, while n847 immunofluorescence and Thioflavin-S double-staining showed that a subset of n847-labeled neurons were Thioflavin-S-positive. In addition, immuno-electron microscopy revealed that tau-positive filaments in tangle-bearing neurons were also labeled by n847 and IHC of other tauopathies showed that some of glial and neuronal tau pathologies in CBD, progressive supranuclear palsy, PiD, and frontotemporal dementia with parkinsonism linked to chromosome 17 also were n847-positive. Finally, nitrated and Thioflavin-S-positive tau aggregates were generated in a oligodendrocytic cell line after treatment with peroxynitrite. Taken together, these findings imply that nitrative injury is directly linked to the formation of filamentous tau inclusions.
Figures


Similar articles
-
Glycogen synthase kinase-3 is associated with neuronal and glial hyperphosphorylated tau deposits in Alzheimer's disease, Pick's disease, progressive supranuclear palsy and corticobasal degeneration.Acta Neuropathol. 2002 Dec;104(6):583-91. doi: 10.1007/s00401-002-0587-8. Epub 2002 Jul 13. Acta Neuropathol. 2002. PMID: 12410379
-
AMPK is abnormally activated in tangle- and pre-tangle-bearing neurons in Alzheimer's disease and other tauopathies.Acta Neuropathol. 2011 Mar;121(3):337-49. doi: 10.1007/s00401-010-0759-x. Epub 2010 Oct 19. Acta Neuropathol. 2011. PMID: 20957377 Free PMC article.
-
Phosphorylated mitogen-activated protein kinase (MAPK/ERK-P), protein kinase of 38 kDa (p38-P), stress-activated protein kinase (SAPK/JNK-P), and calcium/calmodulin-dependent kinase II (CaM kinase II) are differentially expressed in tau deposits in neurons and glial cells in tauopathies.J Neural Transm (Vienna). 2001;108(12):1397-415. doi: 10.1007/s007020100016. J Neural Transm (Vienna). 2001. PMID: 11810404
-
[Neuropathology of tauopathy].Brain Nerve. 2013 Dec;65(12):1445-58. Brain Nerve. 2013. PMID: 24323931 Review. Japanese.
-
Structure of NFT: Biochemical Approach.Adv Exp Med Biol. 2019;1184:23-34. doi: 10.1007/978-981-32-9358-8_2. Adv Exp Med Biol. 2019. PMID: 32096025 Review.
Cited by
-
Neuroprotective effect of 2-(4-methoxyphenyl)ethyl-2-acetamido-2-deoxy-β-D-pyranoside against sodium nitroprusside-induced neurotoxicity in HT22 cells.Mol Cell Biochem. 2013 Nov;383(1-2):149-59. doi: 10.1007/s11010-013-1763-y. Epub 2013 Jul 20. Mol Cell Biochem. 2013. PMID: 23873333
-
Increase in tau tyrosine phosphorylation correlates with the formation of tau aggregates.Brain Res Mol Brain Res. 2005 Aug 18;138(2):135-44. doi: 10.1016/j.molbrainres.2005.04.015. Brain Res Mol Brain Res. 2005. PMID: 15913839 Free PMC article.
-
Naturally Occurring Antioxidant Therapy in Alzheimer's Disease.Antioxidants (Basel). 2022 Jan 23;11(2):213. doi: 10.3390/antiox11020213. Antioxidants (Basel). 2022. PMID: 35204096 Free PMC article. Review.
-
Tau Hyperphosphorylation and Oxidative Stress, a Critical Vicious Circle in Neurodegenerative Tauopathies?Oxid Med Cell Longev. 2015;2015:151979. doi: 10.1155/2015/151979. Epub 2015 Oct 20. Oxid Med Cell Longev. 2015. PMID: 26576216 Free PMC article. Review.
-
Interacting Models of Amyloid-β and Tau Proteins: An Approach to Identify Drug Targets in Alzheimer's Disease.J Alzheimers Dis Rep. 2021 May 14;5(1):405-411. doi: 10.3233/ADR-210018. J Alzheimers Dis Rep. 2021. PMID: 34189412 Free PMC article. Review.
References
-
- Takeda A, Smith MA, Avila J, Nunomura A, Siedlak SL, Zhu X, Perry G, Sayre LM: In Alzheimer’s disease, heme oxygenase is coincident with Alz50, an epitope of tau induced by 4-hydroxy-2-nonenal modification. J Neurochem 2000, 75:1234-1241 - PubMed
-
- Pratico D, Lee VMY, Trojanowski JQ, Rokach J, FitzGerald GA: Increased F2-isoprostanes in Alzheimer’s disease: evidence for enhanced lipid peroxidation in vivo. EMBO J 1998, 12:1777-1783 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

