Complement C5 in experimental autoimmune encephalomyelitis (EAE) facilitates remyelination and prevents gliosis
- PMID: 12937147
- PMCID: PMC1868269
- DOI: 10.1016/S0002-9440(10)63466-9
Complement C5 in experimental autoimmune encephalomyelitis (EAE) facilitates remyelination and prevents gliosis
Abstract
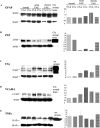
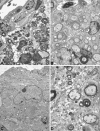
Activation of the classical complement system is known to play a central role in autoimmune demyelination. We have analyzed the role of complement component C5 in experimental autoimmune encephalomyelitis (EAE) using C5-deficient (C5-d) and C5-sufficient (C5-s) mice. Both groups of mice displayed early onset EAE, a short recovery phase, and similar stable chronic courses. However, in contrast to the clinical similarities, marked differences were apparent by histopathology. During acute EAE in C5-d, a delay in inflammatory cell infiltration and tissue damage was observed along with restricted lesion areas, which in C5-s mice were more extensive and diffuse. More striking were the differences in chronic lesions. In C5-d mice, inflammatory demyelination and Wallerian degeneration were followed by axonal depletion and severe gliosis, while in C5-s, the same initial signs were followed by axonal sparing and extensive remyelination. In C5-d, immunohistochemistry and Western blotting showed an increase in glial fibrillary acidic protein and a decrease in neurofilament protein, proteolipid protein, and several pro-inflammatory markers. These results in the EAE model indicate that absence of C5 resulted in fiber loss and extensive scarring, whereas presence of C5-favored axonal survival and more efficient remyelination.
Figures
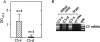



References
-
- Botto M: C1q knockout mice for the study of complement deficiency in autoimmune disease. Exp Clin Immunogenet 1998, 15:231-234 - PubMed
-
- Morgan BP, Walport MJ: Complement deficiency and disease. Immunol Today 1991, 12:301-306 - PubMed
-
- Gasque P, Dean YD, McGreal EP, VanBeek J, Morgan BP: Complement components of the innate immune system in health and disease in the CNS. Immunopharmacology 2000, 49:171-186 - PubMed
-
- Shin M, Rus H, Niculescu F: Complement system in central nervous system disorders. Volankis JE Frank MM eds. The Human Complement in Health and Disease 1998:pp 499-525 Marcel Dekker, New York
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

