NCAM(CD56) and RUNX1(AML1) are up-regulated in human ischemic cardiomyopathy and a rat model of chronic cardiac ischemia
- PMID: 12937148
- PMCID: PMC1868264
- DOI: 10.1016/S0002-9440(10)63467-0
NCAM(CD56) and RUNX1(AML1) are up-regulated in human ischemic cardiomyopathy and a rat model of chronic cardiac ischemia
Abstract
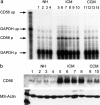
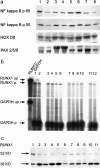
Chronic myocardial ischemia is the leading cause of impaired myocardial contractility and heart failure. To identify differentially expressed genes in human ischemic cardiomyopathy (ICM), we constructed a subtracted cDNA library using specimens of ICM compared to normal human heart. Among 100 randomly sequenced clones, seven sequences represented recently identified candidate genes for differential expression in cardiac hypertrophy. A further clone without a known hypertrophy-association coded for the adhesion molecule NCAM(CD56). RNase protection assay, immunohistochemistry, and Western blotting revealed strong overexpression of NCAM(CD56) in all hearts with ICM (n = 14) compared to normal hearts (n = 8), whereas in congestive cardiomyopathy (CCM) (n = 8), hypertrophic obstructive cardiomyopathy (n = 2), myocarditis (n = 4), and sarcoidosis (n = 2), at most slight overexpression of NCAM(CD56) was observed. NCAM(CD56) overexpression abnormally involved the whole cell membrane and the cytoplasma of cardiomyocytes only inside and adjacent to ischemia-induced cardiac scars. Normal or hypertrophic fibers at a distance from ischemic scars were devoid of NCAM overexpression. Identical alterations were observed in an experimental rat ICM model, but not in normal nor in spontaneously hypertensive rat hearts. In search of NCAM(CD56)-related transcription factors we found RUNX1(AML1) up-regulation in ICM and detected RUNX1(AML1) binding within the NCAM(CD56) promoter by electromobility shift assay. We concluded that strong overexpression of NCAM(CD56) and RUNX1(AML1) is a constant and characteristic feature of cardiomyocytes within or adjacent to scars in ICM.
Figures






References
-
- Gheorghiade M, Bonow RO: Chronic heart failure in the United States: a manifestation of coronary artery disease. Circulation 1998, 97:282-289 - PubMed
-
- Bourassa MG, Gurne O, Bangdiwala SI, Ghali JK, Young JB, Rousseau M, Johnstone DE, Yusuf S: Natural history and patterns of current practice in heart failure: the Studies of Left Ventricular Dysfunction (SOLVD) Investigators. J Am Coll Cardiol 1993, 22:14A-19A - PubMed
-
- Williams JF BM, Fowler MB, Francis GS, Garson A, Jr, Gersh BJ, Hammer DF, Hlatky MA, Leier CV, Packer M, Pitt B, Ullyot DJ, Wexler LF, Winters W, Jr, Ritchie JL, Cheitlin MD, Eagle KA, Gardner TJ, Gibbons RJ, Lewis RP, O’Rourke RA, Ryan TJ: Guidelines for the evaluation and management of heart failure: report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Evaluation and Management of Heart Failure). Circulation 1995, 92:2764.
-
- Richardson P, McKenna W, Bristow M, Maisch B, Mautner B, O’Connell J, Olsen E, Thiene G, Goodwin J, Gyarfas I, Martin I, Nordet P: Report of the 1995 World Health Organization/International Society and Federation of Cardiology Task Force on the definition and classification of cardiomyopathies. Circulation 1996, 93:841-842 - PubMed
-
- Felker GM, Shaw LK, O’Connor CM: A standardized definition of ischemic cardiomyopathy for use in clinical research. J Am Coll Cardiol 2002, 39:210-218 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

