Induction of fatal inflammation in LDL receptor and ApoA-I double-knockout mice fed dietary fat and cholesterol
- PMID: 12937162
- PMCID: PMC1868257
- DOI: 10.1016/S0002-9440(10)63480-3
Induction of fatal inflammation in LDL receptor and ApoA-I double-knockout mice fed dietary fat and cholesterol
Abstract
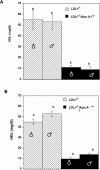
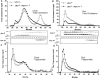
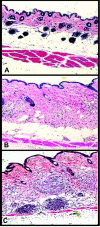
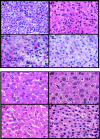
Atherogenic response to dietary fat and cholesterol challenge was evaluated in mice lacking both the LDL receptor (LDLr(-/-)) and apoA-I (apoA-I(-/-)) gene, LDLr(-/-)/apoA-I(-/-) or double-knockout mice. Gender- and age-matched LDLr(-/-)/apoA-I(-/-) mice were fed a diet consisting of 0.1% cholesterol and 10% palm oil for 16 weeks and compared to LDLr(-/-) mice or single-knockout mice. The LDLr(-/-) mice showed a 6- to 7-fold increase in total plasma cholesterol (TPC) compared to their chow-fed mice counterparts, while LDLr(-/-)/apoA-I(-/-) mice showed only a 2- to 3-fold increase in TPC compared to their chow-fed controls. This differential response to the atherogenic diet was unanticipated, since chow-fed LDLr(-/-) and LDLr(-/-)/apoA-I(-/-) mice began the study with similar LDL levels and differed primarily in their HDL concentration. The 6-fold diet-induced increase in TPC observed in the LDLr(-/-) mice occurred mainly in VLDL/LDL and not in HDL. Mid-study plasma samples taken after 8 weeks of diet feeding showed that LDLr(-/-) mice had TPC concentrations approximately 60% of their 16-week level, while the LDLr(-/-)/apoA-I(-/-) mice had reached 100% of their 16-week TPC concentration after only 8 weeks of diet. Male LDLr(-/-) mice showed similar aortic cholesterol levels to male LDLr(-/-)/apoA-I(-/-) mice despite a 4-fold higher VLDL/LDL concentration in the LDLr(-/-) mice. A direct comparison of the severity of aortic atherosclerosis between female LDLr(-/-) and LDLr(-/-)/apoA-I(-/-) mice was compromised due to the loss of female LDLr(-/-)/apoA-I(-/-) mice between 10 and 14 weeks into the study. Diet-fed female and, with time, male LDLr(-/-)/apoA-I(-/-) mice suffered from severe ulcerated cutaneous xanthomatosis. This condition, combined with a complete depletion of adrenal cholesterol, manifested in fatal wasting of the affected mice. In conclusion, LDLr(-/-) and LDLr(-/-)/apoA-I(-/-) mice showed dramatic TPC differences in response to dietary fat and cholesterol challenge, while despite these differences both genotypes accumulated similar levels of aortic cholesterol.
Figures



Similar articles
-
Apolipoprotein A-I deficiency results in markedly increased atherosclerosis in mice lacking the LDL receptor.Arterioscler Thromb Vasc Biol. 2003 Oct 1;23(10):1914-20. doi: 10.1161/01.ATV.0000092328.66882.F5. Epub 2003 Aug 21. Arterioscler Thromb Vasc Biol. 2003. PMID: 12933536
-
Western-type diets induce insulin resistance and hyperinsulinemia in LDL receptor-deficient mice but do not increase aortic atherosclerosis compared with normoinsulinemic mice in which similar plasma cholesterol levels are achieved by a fructose-rich diet.Arterioscler Thromb Vasc Biol. 1999 May;19(5):1223-30. doi: 10.1161/01.atv.19.5.1223. Arterioscler Thromb Vasc Biol. 1999. PMID: 10323773
-
Massive xanthomatosis and atherosclerosis in cholesterol-fed low density lipoprotein receptor-negative mice.J Clin Invest. 1994 May;93(5):1885-93. doi: 10.1172/JCI117179. J Clin Invest. 1994. PMID: 8182121 Free PMC article.
-
Atherogenic hyperlipoproteinemia. The cellular and molecular biology of plasma lipoproteins altered by dietary fat and cholesterol.Med Clin North Am. 1982 Mar;66(2):375-402. doi: 10.1016/s0025-7125(16)31426-2. Med Clin North Am. 1982. PMID: 7040843 Review.
-
The relationship of dietary cholesterol to serum cholesterol concentration and to atherosclerosis in man.Am J Clin Nutr. 1979 Dec;32(12 Suppl):2664-702. doi: 10.1093/ajcn/32.12.2664. Am J Clin Nutr. 1979. PMID: 389024 Review. No abstract available.
Cited by
-
Atherosclerosis: Pathogenesis and Key Cellular Processes, Current and Emerging Therapies, Key Challenges, and Future Research Directions.Methods Mol Biol. 2022;2419:3-19. doi: 10.1007/978-1-0716-1924-7_1. Methods Mol Biol. 2022. PMID: 35237955
-
Lipid-Free Apolipoprotein A-I Reduces Progression of Atherosclerosis by Mobilizing Microdomain Cholesterol and Attenuating the Number of CD131 Expressing Cells: Monitoring Cholesterol Homeostasis Using the Cellular Ester to Total Cholesterol Ratio.J Am Heart Assoc. 2016 Nov 7;5(11):e004401. doi: 10.1161/JAHA.116.004401. J Am Heart Assoc. 2016. PMID: 27821400 Free PMC article.
-
Myeloperoxidase Inhibition Ameliorates Plaque Psoriasis in Mice.Antioxidants (Basel). 2021 Aug 25;10(9):1338. doi: 10.3390/antiox10091338. Antioxidants (Basel). 2021. PMID: 34572970 Free PMC article.
-
Comparative transcriptomic profiling of hydrogen peroxide signaling networks in zebrafish and human keratinocytes: Implications toward conservation, migration and wound healing.Sci Rep. 2016 Feb 5;6:20328. doi: 10.1038/srep20328. Sci Rep. 2016. PMID: 26846883 Free PMC article.
-
Apolipoprotein A-I and its role in lymphocyte cholesterol homeostasis and autoimmunity.Arterioscler Thromb Vasc Biol. 2009 Jun;29(6):843-9. doi: 10.1161/ATVBAHA.108.183442. Epub 2009 Mar 12. Arterioscler Thromb Vasc Biol. 2009. PMID: 19286630 Free PMC article.
References
-
- Boden WE: High-density lipoprotein cholesterol as an independent risk factor in cardiovascular disease: assessing the data from Framingham to the Veterans Affairs high-density lipoprotein intervention trial. Am J Cardiol 2000, 86:19L-22L - PubMed
-
- Gordon DJ, Probstfield JL, Garrison RJ, Neaton JD, Castelli WP, Knoke JD, Jacobs DR, Jr, Bangdiwala S, Tyroler HA: High-density lipoprotein cholesterol and cardiovascular disease: four prospective american studies. Circulation 1989, 79:8-15 - PubMed
-
- Navab M, Van Lenten BJ, Reddy ST, Fogelman AM: High-density lipoprotein and the dynamics of atherosclerotic lesions. Circulation 2001, 104:2386-2387 - PubMed
-
- Walsh A, Ito Y, Breslow JL: High levels of human apolipoprotein A-I in transgenic mice result in increased plasma levels of small high-density lipoprotein (HDL) particles comparable to human HDL3. J Biol Chem 1989, 264:6488-6494 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

