Matrix metalloproteinase 19 regulates insulin-like growth factor-mediated proliferation, migration, and adhesion in human keratinocytes through proteolysis of insulin-like growth factor binding protein-3
- PMID: 12937269
- PMCID: PMC266773
- DOI: 10.1091/mbc.e03-01-0009
Matrix metalloproteinase 19 regulates insulin-like growth factor-mediated proliferation, migration, and adhesion in human keratinocytes through proteolysis of insulin-like growth factor binding protein-3
Abstract
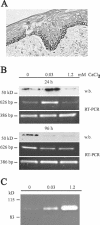
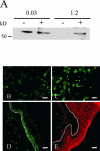
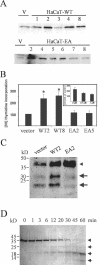
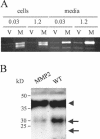
Unlike most other matrix metalloproteinases (MMPs) MMP-19 is expressed in undifferentiated basal keratinocytes of healthy human skin. The human keratinocyte cell line HaCaT, which like basal keratinocytes constitutively expresses MMP-19, down-regulated the expression of MMP-19 at high calcium concentrations. Calcium-regulation occurred through E-cadherin mediated cell-cell contacts because neutralizing anti-E-cadherin antibodies restored MMP-19 expression in high calcium. Overexpression of MMP-19 in HaCaT cells (HaCaT-WT) increased cellular proliferation, as well as migration and adhesion on type I collagen. This was due to proteolysis of the insulin-like growth factor (IGF) binding protein-3 by MMP-19, which augmented signaling through the IGF-I receptor, as evidenced by its increased autophosphorylation. Conversely, these effects were not observed in cells transfected with MMP-2 or a catalytically inactive MMP-19 mutant. As further proof that increased IGF-signaling promoted adhesion and migration in HaCaT-WT cells, we reproduced these effects by treating parental HaCaT with IGF-I. We observed dephosphorylation of the focal adhesion kinase in HaCaT-WT as well as IGF-I-treated HaCaT cells, suggesting that inactivating focal adhesion kinase is a mechanism by which IGF-I enhances adhesion. Furthermore, IGF-I-triggered motility on type I collagen was mediated by MMP activity, which, however, was distinct from MMP-19. Considering the coexpression of IGFBP-3 and MMP-19 in the skin, we conclude that MMP-19 is a likely candidate to be the major IGFBP-3 degrading MMP in the quiescent epidermis. This activity might have widespread consequences for the behavior of epidermal keratinocytes.
Figures


Similar articles
-
Calcium regulates the expression of insulin-like growth factor binding protein-3 by the human keratinocyte cell line HaCaT.J Invest Dermatol. 2001 Apr;116(4):491-7. doi: 10.1046/j.0022-202x.2001.temp.doc.x. J Invest Dermatol. 2001. PMID: 11286613
-
UV induced responses of the human epidermal IGF system: impaired anti-apoptotic effects of IGF-I in HaCaT keratinocytes.Growth Factors. 2005 Jun;23(2):151-9. doi: 10.1080/08977190500153680. Growth Factors. 2005. PMID: 16019437
-
Proprotein convertases regulate insulin-like growth factor 1-induced membrane-type 1 matrix metalloproteinase in VSMCs via endoproteolytic activation of the insulin-like growth factor-1 receptor.Biochem Biophys Res Commun. 2004 Aug 27;321(3):531-8. doi: 10.1016/j.bbrc.2004.07.001. Biochem Biophys Res Commun. 2004. PMID: 15358140
-
Targeting insulin-like growth factor-I and insulin-like growth factor-binding protein-3 signaling pathways. A novel therapeutic approach for asthma.Am J Respir Cell Mol Biol. 2014 Apr;50(4):667-77. doi: 10.1165/rcmb.2013-0397TR. Am J Respir Cell Mol Biol. 2014. PMID: 24219511 Free PMC article. Review.
-
IGF-I receptor, cell-cell adhesion, tumour development and progression.J Mol Histol. 2004 Mar;35(3):247-53. doi: 10.1023/b:hijo.0000032356.98363.a2. J Mol Histol. 2004. PMID: 15339044 Review.
Cited by
-
Gq-coupled purinergic receptors inhibit insulin-like growth factor-I/phosphoinositide 3-kinase pathway-dependent keratinocyte migration.Mol Biol Cell. 2010 Mar 15;21(6):946-55. doi: 10.1091/mbc.e09-06-0497. Epub 2010 Jan 20. Mol Biol Cell. 2010. PMID: 20089844 Free PMC article.
-
Antiangiogenic antitumor activities of IGFBP-3 are mediated by IGF-independent suppression of Erk1/2 activation and Egr-1-mediated transcriptional events.Blood. 2011 Sep 1;118(9):2622-31. doi: 10.1182/blood-2010-08-299784. Epub 2011 May 6. Blood. 2011. PMID: 21551235 Free PMC article.
-
Pharmacoproteomics of a metalloproteinase hydroxamate inhibitor in breast cancer cells: dynamics of membrane type 1 matrix metalloproteinase-mediated membrane protein shedding.Mol Cell Biol. 2008 Aug;28(15):4896-914. doi: 10.1128/MCB.01775-07. Epub 2008 May 27. Mol Cell Biol. 2008. PMID: 18505826 Free PMC article.
-
Diet-induced obesity and reduced skin cancer susceptibility in matrix metalloproteinase 19-deficient mice.Mol Cell Biol. 2004 Jun;24(12):5304-13. doi: 10.1128/MCB.24.12.5304-5313.2004. Mol Cell Biol. 2004. PMID: 15169894 Free PMC article.
-
Polarized monocyte response to cytokine stimulation.Genome Biol. 2005;6(2):R15. doi: 10.1186/gb-2005-6-2-r15. Epub 2005 Jan 21. Genome Biol. 2005. PMID: 15693944 Free PMC article.
References
-
- Ando, Y., and Jensen, P.J. (1993). Epidermal growth factor and insulin-like growth factor I enhance keratinocyte migration. J. Investig. Dermatol. 100, 633-639. - PubMed
-
- Baron, V., Calleja, V., Ferrari, P., Alengrin, F., and van Obberghen, E. (1998). p125FAK focal adhesion kinase is a substrate for the insulin and insulin-like growth factor-I tryrosine kinase receptors. J. Biol. Chem. 273, 7162-7168. - PubMed
-
- Bikle, D.D., and Pillai, S. (1993). Vitamin D, calcium, and epidermal differentiation. Endocr. Rev. 14, 3-19. - PubMed
-
- Birkedahl-Hansen, H., Moore, W.G., Bodden, M.-K., Windsor, L.J., Birkedahl-Hansen, B., DeCarlo, A., and Engler, J.-A. (1993). Matrix metalloproteinases: a review. Crit. Rev. Oral Biol. Med. 4, 197. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

