Maximal polar growth potential depends on the polarisome component AgSpa2 in the filamentous fungus Ashbya gossypii
- PMID: 12937275
- PMCID: PMC207006
- DOI: 10.1091/mbc.e03-03-0167
Maximal polar growth potential depends on the polarisome component AgSpa2 in the filamentous fungus Ashbya gossypii
Abstract
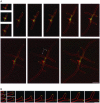
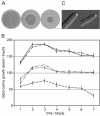
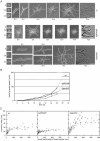
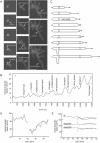
We used actin staining and videomicroscopy to analyze the development from a spore to a young mycelium in the filamentous ascomycete Ashbya gossypii. The development starts with an initial isotropic growth phase followed by the emergence of germ tubes. The initial tip growth speed of 6-10 microm/h increases during early stages of development. This increase is transiently interrupted in response to the establishment of lateral branches or septa. The hyphal tip growth speed finally reaches a maximum of up to 200 micro/h, and the tips of these mature hyphae have the ability to split into two equally fast-growing hyphae. A search for A. gossypii homologs of polarisome components of the yeast Saccharomyces cerevisiae revealed a remarkable size difference between Spa2p of both organisms, with AgSpa2p being double as long as ScSpa2p due to an extended internal domain. AgSpa2 colocalizes with sites of polarized actin. Using time-lapse videomicroscopy, we show that AgSpa2p-GFP polarization is established at sites of branch initiation and then permanently maintained at hyphal tips. Polarization at sites of septation is transient. During apical branching the existing AgSpa2p-GFP polarization is symmetrically divided. To investigate the function of AgSpa2p, we generated two AgSPA2 mutants, a partial deletion of the internal domain alone, and a complete deletion. The mutations had an impact on the maximal hyphal tip growth speed, on the hyphal diameter, and on the branching pattern. We suggest that AgSpa2p is required for the determination of the area of growth at the hyphal tip and that the extended internal domain plays an important role in this process.
Figures



Similar articles
-
A Ras-like GTPase is involved in hyphal growth guidance in the filamentous fungus Ashbya gossypii.Mol Biol Cell. 2004 Oct;15(10):4622-32. doi: 10.1091/mbc.e04-02-0104. Epub 2004 Jul 28. Mol Biol Cell. 2004. PMID: 15282338 Free PMC article.
-
A PAK-like protein kinase is required for maturation of young hyphae and septation in the filamentous ascomycete Ashbya gossypii.J Cell Sci. 2000 Dec;113 Pt 24:4563-75. doi: 10.1242/jcs.113.24.4563. J Cell Sci. 2000. PMID: 11082049
-
The SH3/PH domain protein AgBoi1/2 collaborates with the Rho-type GTPase AgRho3 to prevent nonpolar growth at hyphal tips of Ashbya gossypii.Eukaryot Cell. 2006 Oct;5(10):1635-47. doi: 10.1128/EC.00210-06. Epub 2006 Sep 1. Eukaryot Cell. 2006. PMID: 16950929 Free PMC article.
-
Evolution of multinucleated Ashbya gossypii hyphae from a budding yeast-like ancestor.Fungal Biol. 2011 Jun;115(6):557-68. doi: 10.1016/j.funbio.2011.02.015. Epub 2011 Feb 26. Fungal Biol. 2011. PMID: 21640319 Review.
-
Homologues of yeast polarity genes control the development of multinucleated hyphae in Ashbya gossypii.Curr Opin Microbiol. 2005 Aug;8(4):370-7. doi: 10.1016/j.mib.2005.06.021. Curr Opin Microbiol. 2005. PMID: 16023404 Review.
Cited by
-
Random and direct mutagenesis to enhance protein secretion in Ashbya gossypii.Bioengineered. 2013 Sep-Oct;4(5):322-31. doi: 10.4161/bioe.24653. Epub 2013 Apr 15. Bioengineered. 2013. PMID: 23644277 Free PMC article.
-
Functional characterization of Aspergillus nidulans homologues of Saccharomyces cerevisiae Spa2 and Bud6.Eukaryot Cell. 2006 Jun;5(6):881-95. doi: 10.1128/EC.00036-06. Eukaryot Cell. 2006. PMID: 16757736 Free PMC article.
-
A spindle pole antigen gene MoSPA2 is important for polar cell growth of vegetative hyphae and conidia, but is dispensable for pathogenicity in Magnaporthe oryzae.Curr Genet. 2014 Nov;60(4):255-63. doi: 10.1007/s00294-014-0431-4. Epub 2014 May 25. Curr Genet. 2014. PMID: 24859315
-
Negative feedback equalizes polarity sites in a multi-budding yeast.Curr Biol. 2025 Jul 7;35(13):3022-3034.e4. doi: 10.1016/j.cub.2025.05.011. Epub 2025 Jun 6. Curr Biol. 2025. PMID: 40482649
-
A gene duplication of a septin reveals a developmentally regulated filament length control mechanism.J Cell Biol. 2023 Mar 6;222(3):e202204063. doi: 10.1083/jcb.202204063. Epub 2023 Feb 14. J Cell Biol. 2023. PMID: 36786832 Free PMC article.
References
-
- Altmann-Johl, R., and Philippsen, P. (1996). AgTHR4, a new selection marker for transformation of the filamentous fungus Ashbya gossypii, maps in a four-gene cluster that is conserved between A. gossypii and Saccharomyces cerevisiae. Mol. Gen. Genet. 250, 69–80. - PubMed
-
- Ayad-Durieux, Y., Knechtle, P., Goff, S., Dietrich, F., and Philippsen, P. (2000). A PAK-like protein kinase is required for maturation of young hyphae and septation in the filamentous ascomycete Ashbya gossypii. J. Cell Sci. 113, 4563–4575. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

