Impairing actin filament or syndapin functions promotes accumulation of clathrin-coated vesicles at the apical plasma membrane of acinar epithelial cells
- PMID: 12937279
- PMCID: PMC266760
- DOI: 10.1091/mbc.e03-05-0315
Impairing actin filament or syndapin functions promotes accumulation of clathrin-coated vesicles at the apical plasma membrane of acinar epithelial cells
Abstract
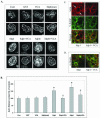
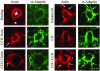
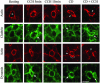
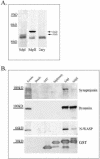
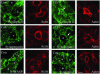
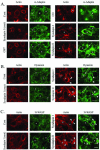
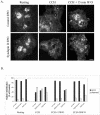
In this article, we investigate the contributions of actin filaments and accessory proteins to apical clathrin-mediated endocytosis in primary rabbit lacrimal acini. Confocal fluorescence and electron microscopy revealed that cytochalasin D promoted apical accumulation of clathrin, alpha-adaptin, dynamin, and F-actin and increased the amounts of coated pits and vesicles at the apical plasma membrane. Sorbitol density gradient analysis of membrane compartments showed that cytochalasin D increased [14C]dextran association with apical membranes from stimulated acini, consistent with functional inhibition of apical endocytosis. Recombinant syndapin SH3 domains interacted with lacrimal acinar dynamin, neuronal Wiskott-Aldrich Syndrome protein (N-WASP), and synaptojanin; their introduction by electroporation elicited remarkable accumulation of clathrin, accessory proteins, and coated pits at the apical plasma membrane. These SH3 domains also significantly (p </= 0.05) increased F-actin, with substantial colocalization of dynamin and N-WASP with the additional filaments. Coelectroporation with the VCA domain of N-WASP blocked the increase in F-actin and reversed the morphological changes indicative of impaired apical endocytosis. We suggest that transient modulation of actin polymerization by syndapins through activation of the Arp2/3 complex via N-WASP coordinates dynamin-mediated vesicle fission at the apical plasma membrane of acinar epithelia. Trapping of assembled F-actin intermediates during this process by cytochalasin D or syndapin SH3 domains impairs endocytosis.
Figures



Similar articles
-
Syndapins integrate N-WASP in receptor-mediated endocytosis.EMBO J. 2002 Nov 15;21(22):6083-94. doi: 10.1093/emboj/cdf604. EMBO J. 2002. PMID: 12426380 Free PMC article.
-
The syndapin protein family: linking membrane trafficking with the cytoskeleton.J Cell Sci. 2004 Jul 1;117(Pt 15):3077-86. doi: 10.1242/jcs.01290. J Cell Sci. 2004. PMID: 15226389 Review.
-
Neural Wiskott Aldrich Syndrome Protein (N-WASP) and the Arp2/3 complex are recruited to sites of clathrin-mediated endocytosis in cultured fibroblasts.Eur J Cell Biol. 2004 Feb;83(1):13-8. doi: 10.1078/0171-9335-00356. Eur J Cell Biol. 2004. PMID: 15085951
-
Syndapin isoforms participate in receptor-mediated endocytosis and actin organization.J Cell Biol. 2000 Mar 6;148(5):1047-62. doi: 10.1083/jcb.148.5.1047. J Cell Biol. 2000. PMID: 10704453 Free PMC article.
-
Coupling actin dynamics and membrane dynamics during endocytosis.Curr Opin Cell Biol. 2002 Feb;14(1):76-81. doi: 10.1016/s0955-0674(01)00297-6. Curr Opin Cell Biol. 2002. PMID: 11792548 Review.
Cited by
-
Transduced viral IL-10 is exocytosed from lacrimal acinar secretory vesicles in a myosin-dependent manner in response to carbachol.Exp Eye Res. 2009 Mar;88(3):467-78. doi: 10.1016/j.exer.2008.10.023. Epub 2008 Nov 13. Exp Eye Res. 2009. PMID: 19056381 Free PMC article.
-
Syndapin/SDPN-1 is required for endocytic recycling and endosomal actin association in the C. elegans intestine.Mol Biol Cell. 2016 Sep 14;27(23):3746-56. doi: 10.1091/mbc.E16-02-0116. Online ahead of print. Mol Biol Cell. 2016. PMID: 27630264 Free PMC article.
-
MICAL-L1-related and unrelated mechanisms underlying elongated tubular endosomal network (ETEN) in human dendritic cells.Commun Integr Biol. 2014 Dec 23;7(6):e994969. doi: 10.4161/19420889.2014.994969. eCollection 2014 Dec. Commun Integr Biol. 2014. PMID: 26478765 Free PMC article.
-
Association with membrane protrusions makes ErbB2 an internalization-resistant receptor.Mol Biol Cell. 2004 Apr;15(4):1557-67. doi: 10.1091/mbc.e03-08-0596. Epub 2004 Jan 23. Mol Biol Cell. 2004. PMID: 14742716 Free PMC article.
-
Protein Delivery to Cytosol by Cell-Penetrating Peptide Bearing Tandem Repeat Penetration-Accelerating Sequence.Methods Mol Biol. 2022;2383:265-273. doi: 10.1007/978-1-0716-1752-6_18. Methods Mol Biol. 2022. PMID: 34766296
References
-
- Apodaca, G. (2001). Endocytic traffic in polarized epithelial cells: role of the actin and microtubule cytoskeleton. Traffic 2, 149-159. - PubMed
-
- Aronson, P.S. (1978). Energy-dependence of phlorizin binding to isolated renal microvillus membranes. Evidence concerning the mechanism of coupling between the electrochemical Na+ gradient and sugar transport. J. Membr. Biol. 42, 81-98. - PubMed
-
- Brodsky, F.M., Chen, C-Y., Knuehl, C., Towler, M.C., and Wakeham, D.E. (2001). Biological basket-weaving: formation and function of clathrin-coated vesicles. Annu. Rev. Cell Dev. Biol. 17, 517-568. - PubMed
-
- Cremona, O., et al. (1999). Essential role of phosphoinositide metabolism in synaptic vesicle recycling. Cell 99, 179-188. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK056040/DK/NIDDK NIH HHS/United States
- NS-38246/NS/NINDS NIH HHS/United States
- R56 EY010550/EY/NEI NIH HHS/United States
- F31 EY007037/EY/NEI NIH HHS/United States
- 1 P30DK48522/DK/NIDDK NIH HHS/United States
- EY-07037/EY/NEI NIH HHS/United States
- R01 GM059297/GM/NIGMS NIH HHS/United States
- EY-11386/EY/NEI NIH HHS/United States
- R01 EY010550/EY/NEI NIH HHS/United States
- R01 NS038246/NS/NINDS NIH HHS/United States
- R01 EY011386/EY/NEI NIH HHS/United States
- EU-05081/PHS HHS/United States
- GM-59297/GM/NIGMS NIH HHS/United States
- DK-56040/DK/NIDDK NIH HHS/United States
- P30 DK048522/DK/NIDDK NIH HHS/United States
- EY-10550/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources

