Role of inhibitory amino acids in control of hypoglossal motor outflow to genioglossus muscle in naturally sleeping rats
- PMID: 12937280
- PMCID: PMC2343458
- DOI: 10.1113/jphysiol.2003.052357
Role of inhibitory amino acids in control of hypoglossal motor outflow to genioglossus muscle in naturally sleeping rats
Abstract
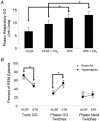
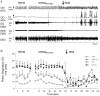
The hypoglossal motor nucleus innervates the genioglossus (GG) muscle of the tongue, a muscle that helps maintain an open airway for effective breathing. Rapid-eye-movement (REM) sleep, however, recruits powerful neural mechanisms that can abolish GG activity even during strong reflex stimulation such as by hypercapnia, effects that can predispose to sleep-related breathing problems in humans. We have developed an animal model to chronically manipulate neurotransmission at the hypoglossal motor nucleus using in vivo microdialysis in freely behaving rats. This study tests the hypothesis that glycine receptor antagonism at the hypoglossal motor nucleus, either alone or in combination with GABAA receptor antagonism, will prevent suppression of GG activity in natural REM sleep during room air and CO2-stimulated breathing. Rats were implanted with electroencephalogram and neck muscle electrodes to record sleep-wake states, and GG and diaphragm electrodes for respiratory muscle recording. Microdialysis probes were implanted into the hypoglossal motor nucleus for perfusion of artificial cerebrospinal fluid (ACSF) and strychnine (glycine receptor antagonist, 0.1 mM) either alone or combined with bicuculline (GABAA antagonist, 0.1 mM) during room air and CO2-stimulated breathing. Compared to ACSF controls, glycine receptor antagonism at the hypoglossal motor nucleus increased respiratory-related GG activity in room air (P = 0.010) but not hypercapnia (P = 0.221). This stimulating effect of strychnine in room air did not depend on the prevailing sleep-wake state (P = 0.625) indicating removal of a non-specific background inhibitory glycinergic tone. Nevertheless, GG activity remained minimal in those REM sleep periods without phasic twitches in GG muscle, with GG suppression from non-REM (NREM) sleep being > 85 % whether ACSF or strychnine was at the hypoglossal motor nucleus or the inspired gas was room air or 7 % CO2. While GG activity was minimal in these REM sleep periods, there was a small but measurable increase in GG activity after strychnine (P < 0.05). GG activity was also minimal, and effectively abolished, in the REM sleep periods without GG twitches with combined glycine and GABAA receptor antagonism at the hypoglossal motor nucleus. We conclude that these data in freely behaving rats confirm that inhibitory glycine and GABAA receptor mechanisms are present at the hypoglossal motor nucleus and are tonically active, but that such inhibitory mechanisms make only a small contribution to the marked suppression of GG activity and reflex responses observed in periods of natural REM sleep.
Figures








References
-
- Bellingham MC, Berger AJ. Presynaptic depression of excitatory synaptic inputs to rat hypoglossal motoneurons by muscarinic M2 receptors. J Neurophysiol. 1996;76:3758–3770. - PubMed
-
- Berger AJ. Determinants of respiratory motoneuron output. Respir Physiol. 2000;122:259–269. - PubMed
-
- Chamberlin NL, Bocchiaro CM, Greene RW, Feldman JL. Nicotinic excitation of rat hypoglossal motoneurons. Neuroscience. 2002;115:861–870. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

