Contractile effects of the exchange of cardiac troponin for fast skeletal troponin in rabbit psoas single myofibrils
- PMID: 12937281
- PMCID: PMC2343446
- DOI: 10.1113/jphysiol.2003.051615
Contractile effects of the exchange of cardiac troponin for fast skeletal troponin in rabbit psoas single myofibrils
Abstract
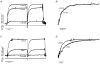
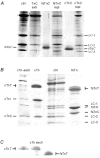
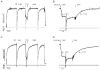
The effects of the removal of fast skeletal troponin C (fsTnC) and its replacement by cardiac troponin C (cTnC) and the exchange of fast skeletal troponin (fsTn) for cardiac troponin (cTn) were measured in rabbit fast skeletal myofibrils. Electrophoretic analysis of myofibril suspensions indicated that replacement of fsTnC or exchange of fsTn with cTnC or cTn was about 90% complete in the protocols used. Mechanical measurements in single myofibrils, which were maximally activated by fast solution switching, showed that replacement of fsTnC with cTnC reduced the isometric tension, the rate of tension rise following a step increase in Ca2+ (kACT), and the rate of tension redevelopment following a quick release and restretch (kTR), but had no effect on the kinetics of the fall in tension when the concentration of inorganic phosphate (Pi) was abruptly increased (kPi(+)). These data suggest that the chimeric protein produced by cTnC replacement in fsTn alters those steps controlling the weak-to-strong crossbridge attachment transition. Inefficient signalling within the chimeric troponin may cause these changes. However, replacement of fsTn by cTn had no effect on maximal isometric tension, kACT or kTR, suggesting that these mechanics are largely determined by the isoform of the myosin molecule. Replacement of fsTn by cTn, on the other hand, shifted the pCa50 of the pCa-tension relationship from 5.70 to 6.44 and reduced the Hill coefficient from 3.3 to 1.4, suggesting that regulatory protein isoforms primarily alter Ca2+ sensitivity and the cooperativity of the force-generating mechanism.
Figures

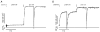
Similar articles
-
Isoform specific interactions of troponin I and troponin C determine pH sensitivity of myofibrillar Ca2+ activation.Biochemistry. 1994 Jul 19;33(28):8464-71. doi: 10.1021/bi00194a010. Biochemistry. 1994. PMID: 8031779
-
Calcium binding kinetics of troponin C strongly modulate cooperative activation and tension kinetics in cardiac muscle.J Mol Cell Cardiol. 2011 Jan;50(1):165-74. doi: 10.1016/j.yjmcc.2010.10.025. Epub 2010 Oct 28. J Mol Cell Cardiol. 2011. PMID: 21035455 Free PMC article.
-
Changes in myofibrillar activation and troponin C Ca2+ binding associated with troponin T isoform switching in developing rabbit heart.Circ Res. 1990 May;66(5):1204-16. doi: 10.1161/01.res.66.5.1204. Circ Res. 1990. PMID: 2139820
-
Isometric force redevelopment of skinned muscle fibers from rabbit activated with and without Ca2+.Biophys J. 1994 Nov;67(5):1994-2001. doi: 10.1016/S0006-3495(94)80682-4. Biophys J. 1994. PMID: 7858136 Free PMC article.
-
Mechanism of cross-bridge detachment in isometric force relaxation of skeletal and cardiac myofibrils.J Muscle Res Cell Motil. 2003;24(4-6):261-7. J Muscle Res Cell Motil. 2003. PMID: 14620739 Review.
Cited by
-
A re-interpretation of the rate of tension redevelopment (k(TR)) in active muscle.J Muscle Res Cell Motil. 2013 Dec;34(5-6):407-15. doi: 10.1007/s10974-013-9366-5. Epub 2013 Oct 27. J Muscle Res Cell Motil. 2013. PMID: 24162314 Free PMC article.
-
Troponin and titin coordinately regulate length-dependent activation in skinned porcine ventricular muscle.J Gen Physiol. 2008 Mar;131(3):275-83. doi: 10.1085/jgp.200709895. J Gen Physiol. 2008. PMID: 18299397 Free PMC article.
-
The relation between sarcomere energetics and the rate of isometric tension relaxation in healthy and diseased cardiac muscle.J Muscle Res Cell Motil. 2021 Mar;42(1):47-57. doi: 10.1007/s10974-019-09566-2. Epub 2019 Nov 19. J Muscle Res Cell Motil. 2021. PMID: 31745760 Free PMC article.
-
Mechanical and kinetic effects of shortened tropomyosin reconstituted into myofibrils.Pflugers Arch. 2009 Aug;458(4):761-76. doi: 10.1007/s00424-009-0653-3. Epub 2009 Mar 3. Pflugers Arch. 2009. PMID: 19255776 Free PMC article.
-
Insights into the kinetics of Ca2+-regulated contraction and relaxation from myofibril studies.Pflugers Arch. 2009 Jun;458(2):337-57. doi: 10.1007/s00424-008-0630-2. Epub 2009 Jan 23. Pflugers Arch. 2009. PMID: 19165498
References
-
- Babu A, Scordilis SP, Sonnenblick EH, Gulati J. The control of myocardial contraction with skeletal fast muscle troponin. J Biol Chem. 1987;262:5815–5822. - PubMed
-
- Brandt PW, Diamond MS, Schachat FH. The thin filament of vertebrate skeletal muscle cooperatively activates as a unit. J Mol Biol. 1984;180:379–384. - PubMed
-
- Butters CA, Tobacman JB, Tobacman LS. Cooperative effect of calcium binding to adjacent troponin molecules on the thin filament-myosin subfragment 1 MgATPase rate. J Biol Chem. 1997;272:13196–13202. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

