P2X2 subunits contribute to fast synaptic excitation in myenteric neurons of the mouse small intestine
- PMID: 12937291
- PMCID: PMC2343442
- DOI: 10.1113/jphysiol.2003.047944
P2X2 subunits contribute to fast synaptic excitation in myenteric neurons of the mouse small intestine
Abstract
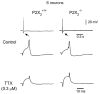
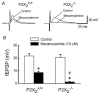
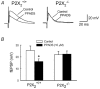
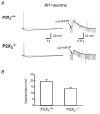
P2X receptors are ATP-gated cation channels composed of one or more of seven different subunits. ATP acts at P2X receptors to contribute to fast excitatory postsynaptic potentials (fEPSPs) in myenteric neurons but the subunit composition of enteric P2X receptors is unknown. These studies used tissues from P2X2 wild-type (P2X2+/+) and P2X2 gene knockout (P2X2-/-) mice to investigate the role of this subunit in enteric neurotransmission. Intracellular electrophysiological methods were used to record synaptic and drug-induced responses from ileal myenteric neurons in vitro. Drug-induced longitudinal muscle contractions and peristaltic contractions of ileal segments were also studied in vitro. Gastrointestinal transit was measured as the progression in 30 min of a liquid radioactive marker administered by gavage to fasted mice. RT-PCR analysis of mRNA from intestinal tissues and data from immunohistochemical studies verified P2X2 gene deletion. The fEPSPs recorded from S neurons in tissues from P2X2+/+ mice were reduced by mecamylamine (nicotinic cholinergic receptor antagonist) and PPADS (P2X receptor antagonist). The fEPSPs recorded from S neurons from P2X2-/- mice were unaffected by PPADS but were blocked by mecamylamine. ATP depolarized S and AH neurons from P2X2+/+ mice. ATP depolarized AH but not S neurons from P2X2-/- mice. alpha,beta-Methylene ATP (alpha,beta-mATP)(an agonist at P2X3 subunit-containing receptors) did not depolarize S neurons but it did depolarize AH neurons in P2X2+/+ and P2X2-/- mice. Peristalsis was inhibited in ileal segments from P2X2-/- mice but longitudinal muscle contractions caused by nicotine and bethanechol were similar in segments from P2X2+/+ and P2X2-/- mice. Gastrointestinal transit was similar in P2X2+/+ and P2X2-/- mice. It is concluded that P2X2 homomeric receptors contribute to fEPSPs in neural pathways underlying peristalsis studied in vitro.
Figures




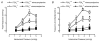
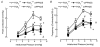
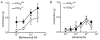
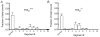
References
-
- Abdu F, Hicks GA, Hennig G, Allen JP, Grundy D. Somatostatin sst(2) receptors inhibit peristalsis in the rat and mouse jejunum. Am J Physiol. 2002;282:G624–633. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

