Immediate response of mammalian target of rapamycin (mTOR)-mediated signalling following acute resistance exercise in rat skeletal muscle
- PMID: 12937293
- PMCID: PMC2343483
- DOI: 10.1113/jphysiol.2003.047019
Immediate response of mammalian target of rapamycin (mTOR)-mediated signalling following acute resistance exercise in rat skeletal muscle
Abstract
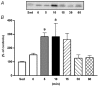
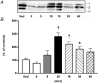
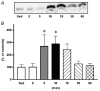
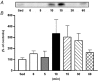
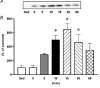
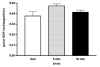
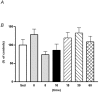
The purpose of the present investigation was to determine whether mammalian target of rapamycin (mTOR)-mediated signalling and some key regulatory proteins of translation initiation are altered in skeletal muscle during the immediate phase of recovery following acute resistance exercise. Rats were operantly conditioned to reach an illuminated bar located high on a Plexiglass cage, such that the animals completed concentric and eccentric contractions involving the hindlimb musculature. Gastrocnemius muscle was extracted immediately after acute exercise and 5, 10, 15, 30 and 60 min of recovery. Phosphorylation of protein kinase B (PKB) on Ser-473 peaked at 10 min of recovery (282% of control, P < 0.05) with no significant changes noted for mTOR phosphorylation on Ser-2448. Eukaryotic initiation factor (eIF) 4E-binding protein-1 (4E-BP1) and S6 kinase-1 (S6K1), both downstream effectors of mTOR, were altered during recovery as well. 4E-BP1 phosphorylation was significantly elevated at 10 min (292%, P < 0.01) of recovery. S6K1 phosphorylation on Thr-389 demonstrated a trend for peak activation at 10 min following exercise (336%, P = 0.06) with ribosomal protein S6 phosphorylation being maximally activated at 15 min of recovery (647%, P < 0.05). Components of the eIF4F complex were enhanced during recovery as eIF4E association with eIF4G peaked at 10 min (292%, P < 0.05). Events regulating the binding of initiator methionyl-tRNA to the 40S ribosomal subunit were assessed through eIF2B activity and eIF2 alpha phosphorylation on Ser-51. No differences were noted with either eIF2B or eIF2 alpha. Collectively, these results provide strong evidence that mTOR-mediating signalling is transiently upregulated during the immediate period following resistance exercise and this response may constitute the most proximal growth response of the cell.
Figures


Comment in
-
Connecting the dots for mechanochemical transduction in muscle.J Physiol. 2003 Nov 15;553(Pt 1):1. doi: 10.1113/jphysiol.2003.054197. Epub 2003 Sep 8. J Physiol. 2003. PMID: 12963787 Free PMC article.
References
-
- Baar K, Esser KA. Phosphorylation of p70S6k correlates with increased skeletal muscle mass following resistance exercise. Am J Physiol. 1999;276:C120–127. - PubMed
-
- Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, Zlotchenko E, Scrimgeour A, Lawrence JC, Glass DJ, Yancopoulous GD. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nature Cell Biol. 2001;3:1014–1019. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

