Sodium and calcium currents shape action potentials in immature mouse inner hair cells
- PMID: 12937295
- PMCID: PMC2343463
- DOI: 10.1113/jphysiol.2003.043612
Sodium and calcium currents shape action potentials in immature mouse inner hair cells
Abstract
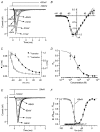
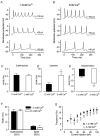
Before the onset of hearing at postnatal day 12, mouse inner hair cells (IHCs) produce spontaneous and evoked action potentials. These spikes are likely to induce neurotransmitter release onto auditory nerve fibres. Since immature IHCs express both alpha1D (Cav1.3) Ca2+ and Na+ currents that activate near the resting potential, we examined whether these two conductances are involved in shaping the action potentials. Both had extremely rapid activation kinetics, followed by fast and complete voltage-dependent inactivation for the Na+ current, and slower, partially Ca2+-dependent inactivation for the Ca2+ current. Only the Ca2+ current is necessary for spontaneous and induced action potentials, and 29 % of cells lacked a Na+ current. The Na+ current does, however, shorten the time to reach the action-potential threshold, whereas the Ca2+ current is mainly involved, together with the K+ currents, in determining the speed and size of the spikes. Both currents increased in size up to the end of the first postnatal week. After this, the Ca2+ current reduced to about 30 % of its maximum size and persisted in mature IHCs. The Na+ current was downregulated around the onset of hearing, when the spiking is also known to disappear. Although the Na+ current was observed as early as embryonic day 16.5, its role in action-potential generation was only evident from just after birth, when the resting membrane potential became sufficiently negative to remove a sizeable fraction of the inactivation (half inactivation was at -71 mV). The size of both currents was positively correlated with the developmental change in action-potential frequency.
Figures







References
-
- Brehm P, Eckert R. Calcium entry leads to inactivation of calcium channel in Paramecium. Science. 1978;202:1203–1206. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

