WNT7a induces E-cadherin in lung cancer cells
- PMID: 12937339
- PMCID: PMC193578
- DOI: 10.1073/pnas.1734137100
WNT7a induces E-cadherin in lung cancer cells
Abstract
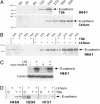
E-cadherin loss in cancer is associated with de-differentiation, invasion, and metastasis. Drosophila DE-cadherin is regulated by Wnt/beta-catenin signaling, although this has not been demonstrated in mammalian cells. We previously reported that expression of WNT7a, encoded on 3p25, was frequently downregulated in lung cancer, and that loss of E-cadherin or beta-catenin was a poor prognostic feature. Here we show that WNT7a both activates E-cadherin expression via a beta-catenin specific mechanism in lung cancer cells and is involved in a positive feedback loop. Li+, a GSK3 beta inhibitor, led to E-cadherin induction in an inositol-independent manner. Similarly, exposure to mWNT7a specifically induced free beta-catenin and E-cadherin. Among known transcriptional suppressors of E-cadherin, ZEB1 was uniquely correlated with E-cadherin loss in lung cancer cell lines, and its inhibition by RNA interference resulted in E-cadherin induction. Pharmacologic reversal of E-cadherin and WNT7a losses was achieved with Li+, histone deacetylase inhibition, or in some cases only with combined inhibitors. Our findings provide support that E-cadherin induction by WNT/beta-catenin signaling is an evolutionarily conserved pathway operative in lung cancer cells, and that loss of WNT7a expression may be important in lung cancer development or progression by its effects on E-cadherin.
Figures


References
-
- Wistuba, I. I., Behrens, C., Virmani, A. K., Mele, G., Milchgrub, S., Girard, L., Fondon, J. W., III, Garner, H. R., McKay, B., Latif, F., et al. (2000) Cancer Res. 60, 1949-1960. - PubMed
-
- Tomizawa, Y., Kohno, T., Kondo, H., Otsuka, A., Nishioka, M., Niki, T., Yamada, T., Maeshima, A., Yoshimura, K., Saito, R., et al. (2002) Clin. Cancer Res. 8, 2362-2368. - PubMed
-
- Tse, C., Xiang, R. H., Bracht, T. & Naylor, S. L. (2002) Cancer Res. 62, 542-546. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

