Activation-dependent properties of pregnenolone sulfate inhibition of GABAA receptor-mediated current
- PMID: 12938673
- PMCID: PMC2343070
- DOI: 10.1113/jphysiol.2003.043810
Activation-dependent properties of pregnenolone sulfate inhibition of GABAA receptor-mediated current
Abstract
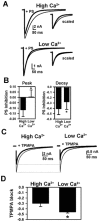
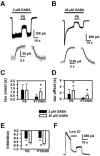
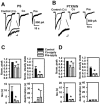
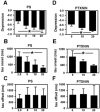
Sulfated steroids like pregnenolone sulfate (PS) are found endogenously in the central nervous system where they may modulate GABAA receptors. Understanding the mechanism of steroid inhibition is important for understanding the conditions under which endogenous steroids modulate GABAA receptor function, assessing their potential clinical utility, and for evaluating sulfated steroids as probes of receptor behaviour. Some previous studies suggest that sulfated steroid inhibition exhibits activation dependence, whilst other studies suggest only slow, time-dependent inhibition, perhaps reflecting slow PS association with receptors. We tested activation dependence in several ways. Steroid potency increased 2- to 3-fold with approximately 10-fold change in GABA concentration. PS inhibition of saturating partial agonist responses suggested that the level of channel activation, rather than receptor occupancy by agonist, is important for PS inhibition. Inhibition by sulfated steroids exhibited weak or no voltage dependence. Responses to rapid applications of exogenous GABA differed little whether PS was pre-applied or simply co-applied with GABA, consistent with the hypothesis that the actions of PS are facilitated by receptor activation. PS applied during steady-state GABA responses exhibited slow onset and offset rate constants. The offset, rather than onset, was significantly slowed by elevated GABA concentration. At hippocampal synapses, large, multiquantal IPSCs were inhibited more effectively by a fixed concentration of PS than small quantal content IPSCs, consistent with known 'pooling' of transmitter following multiquantal release. Picrotoxinin, although superficially similar to PS in its activation dependence, was dissimilar from PS in a number of details. In summary, PS inhibition exhibits activation dependence that may be explained by activation-dependent binding and altered desensitization.
Figures

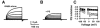


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

