Stroke-induced immunodeficiency promotes spontaneous bacterial infections and is mediated by sympathetic activation reversal by poststroke T helper cell type 1-like immunostimulation
- PMID: 12939340
- PMCID: PMC2194193
- DOI: 10.1084/jem.20021098
Stroke-induced immunodeficiency promotes spontaneous bacterial infections and is mediated by sympathetic activation reversal by poststroke T helper cell type 1-like immunostimulation
Abstract
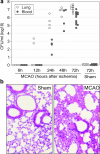
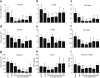
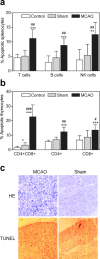
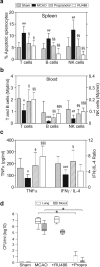
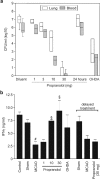
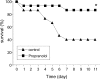
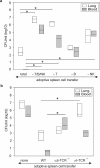
Infections are a leading cause of death in stroke patients. In a mouse model of focal cerebral ischemia, we tested the hypothesis that a stroke-induced immunodeficiency increases the susceptibility to bacterial infections. 3 d after ischemia, all animals developed spontaneous septicemia and pneumonia. Stroke induced an extensive apoptotic loss of lymphocytes and a shift from T helper cell (Th)1 to Th2 cytokine production. Adoptive transfer of T and natural killer cells from wild-type mice, but not from interferon (IFN)-gamma-deficient mice, or administration of IFN-gamma at day 1 after stroke greatly decreased the bacterial burden. Importantly, the defective IFN-gamma response and the occurrence of bacterial infections were prevented by blocking the sympathetic nervous system but not the hypothalamo-pituitary-adrenal axis. Furthermore, administration of the beta-adrenoreceptor blocker propranolol drastically reduced mortality after stroke. These data suggest that a catecholamine-mediated defect in early lymphocyte activation is the key factor in the impaired antibacterial immune response after stroke.
Figures

Comment in
-
Effects of stroke beyond the brain.Nat Rev Immunol. 2019 Dec;19(12):719. doi: 10.1038/s41577-019-0234-4. Nat Rev Immunol. 2019. PMID: 31624320 No abstract available.
References
-
- Davenport, R.J., M.S. Dennis, I. Wellwood, and C.P. Warlow. 1996. Complications after acute stroke. Stroke. 27:415–420. - PubMed
-
- Johnston, K.C., J.Y. Li, P.D. Lyden, S.K. Hanson, T.E. Feasby, R.J. Adams, R.E. Faught, Jr., and E.C. Haley, Jr. 1998. Medical and neurological complications of ischemic stroke: experience from the RANTTAS trial. RANTTAS investigators. Stroke. 29:447–453. - PubMed
-
- Georgilis, K., A. Plomaritoglou, U. Dafni, Y. Bassiakos, and K. Vemmos. 1999. Aetiology of fever in patients with acute stroke. J. Intern. Med. 246:203–209. - PubMed
-
- Grau, A.J., F. Buggle, P. Schnitzler, M. Spiel, C. Lichy, and W. Hacke. 1999. Fever and infection early after ischemic stroke. J. Neurol. Sci. 171:115–120. - PubMed
-
- Langhorne, P., D.J. Stott, L. Robertson, J. MacDonald, L. Jones, C. McAlpine, F. Dick, G.S. Taylor, and G. Murray. 2000. Medical complications after stroke: a multicenter study. Stroke. 31:1223–1229. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

