Carboxyl-terminal-truncated apolipoprotein E4 causes Alzheimer's disease-like neurodegeneration and behavioral deficits in transgenic mice
- PMID: 12939405
- PMCID: PMC196910
- DOI: 10.1073/pnas.1434398100
Carboxyl-terminal-truncated apolipoprotein E4 causes Alzheimer's disease-like neurodegeneration and behavioral deficits in transgenic mice
Abstract
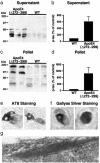
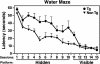
Apolipoprotein (apo) E4 increases the risk and accelerates the onset of Alzheimer's disease (AD). However, the underlying mechanisms remain to be determined. We previously found that apoE undergoes proteolytic cleavage in AD brains and in cultured neuronal cells, resulting in the accumulation of carboxyl-terminal-truncated fragments of apoE that are neurotoxic. Here we show that this fragmentation is caused by proteolysis of apoE by a chymotrypsin-like serine protease that cleaves apoE4 more efficiently than apoE3. Transgenic mice expressing the carboxyl-terminal-cleaved product, apoE4(Delta272-299), at high levels in the brain died at 2-4 months of age. The cortex and hippocampus of these mice displayed AD-like neurodegenerative alterations, including abnormally phosphorylated tau (p-tau) and Gallyas silver-positive neurons that contained cytosolic straight filaments with diameters of 15-20 nm, resembling preneurofibrillary tangles. Transgenic mice expressing lower levels of the truncated apoE4 survived longer but showed impaired learning and memory at 6-7 months of age. Thus, carboxyl-terminal-truncated fragments of apoE4, which occur in AD brains, are sufficient to elicit AD-like neurodegeneration and behavioral deficits in vivo. Inhibiting their formation might inhibit apoE4-associated neuronal deficits.
Figures



Similar articles
-
Neuron-specific apolipoprotein e4 proteolysis is associated with increased tau phosphorylation in brains of transgenic mice.J Neurosci. 2004 Mar 10;24(10):2527-34. doi: 10.1523/JNEUROSCI.4315-03.2004. J Neurosci. 2004. PMID: 15014128 Free PMC article.
-
C-terminal-truncated apolipoprotein (apo) E4 inefficiently clears amyloid-beta (Abeta) and acts in concert with Abeta to elicit neuronal and behavioral deficits in mice.Proc Natl Acad Sci U S A. 2011 Mar 8;108(10):4236-41. doi: 10.1073/pnas.1018381108. Epub 2011 Feb 22. Proc Natl Acad Sci U S A. 2011. PMID: 21368138 Free PMC article.
-
Apolipoprotein E fragments present in Alzheimer's disease brains induce neurofibrillary tangle-like intracellular inclusions in neurons.Proc Natl Acad Sci U S A. 2001 Jul 17;98(15):8838-43. doi: 10.1073/pnas.151254698. Epub 2001 Jul 10. Proc Natl Acad Sci U S A. 2001. PMID: 11447277 Free PMC article.
-
Apolipoprotein (apo) E4 and Alzheimer's disease: unique conformational and biophysical properties of apoE4 can modulate neuropathology.Acta Neurol Scand Suppl. 2006;185:8-14. doi: 10.1111/j.1600-0404.2006.00679.x. Acta Neurol Scand Suppl. 2006. PMID: 16866905 Review.
-
Apolipoprotein E isoforms in Alzheimer's disease pathology and etiology.Microsc Res Tech. 2000 Aug 15;50(4):278-81. doi: 10.1002/1097-0029(20000815)50:4<278::AID-JEMT5>3.0.CO;2-T. Microsc Res Tech. 2000. PMID: 10936880 Review.
Cited by
-
Proteolytic cleavage of apolipoprotein E4 as the keystone for the heightened risk associated with Alzheimer's disease.Int J Mol Sci. 2013 Jul 17;14(7):14908-22. doi: 10.3390/ijms140714908. Int J Mol Sci. 2013. PMID: 23867607 Free PMC article. Review.
-
Central Nervous System Lipoproteins: ApoE and Regulation of Cholesterol Metabolism.Arterioscler Thromb Vasc Biol. 2016 Jul;36(7):1305-15. doi: 10.1161/ATVBAHA.116.307023. Epub 2016 May 12. Arterioscler Thromb Vasc Biol. 2016. PMID: 27174096 Free PMC article. Review.
-
Profile and regulation of apolipoprotein E (ApoE) expression in the CNS in mice with targeting of green fluorescent protein gene to the ApoE locus.J Neurosci. 2006 May 10;26(19):4985-94. doi: 10.1523/JNEUROSCI.5476-05.2006. J Neurosci. 2006. PMID: 16687490 Free PMC article.
-
An Integrative Overview of Non-Amyloid and Non-Tau Pathologies in Alzheimer's Disease.Neurochem Res. 2019 Jan;44(1):12-21. doi: 10.1007/s11064-018-2603-y. Epub 2018 Aug 6. Neurochem Res. 2019. PMID: 30084096 Free PMC article. Review.
-
Effects of toxic apolipoprotein E fragments on Tau phosphorylation and cognitive impairment in neonatal mice under sevoflurane anesthesia.Brain Behav. 2022 Aug;12(8):e2702. doi: 10.1002/brb3.2702. Epub 2022 Jul 10. Brain Behav. 2022. PMID: 35810473 Free PMC article.
References
-
- Mahley, R. W. (1988) Science 240, 622–630. - PubMed
-
- Mahley, R. W. & Huang, Y. (1999) Curr. Opin. Lipidol. 10, 207–217. - PubMed
-
- Huang, Y. & Mahley, R. W. (1999) in Plasma Lipids and Their Role in Disease, eds. Barter, P. J. & Rye, K.-A. (Harwood Academic, Amsterdam), pp. 257–284.
-
- Mahley, R. W. & Rall, S. C., Jr. (2000) Annu. Rev. Genomics Hum. Genet. 1, 507–537. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

