Enhanced mucosal and systemic immune responses to Helicobacter pylori antigens through mucosal priming followed by systemic boosting immunizations
- PMID: 12941145
- PMCID: PMC1783019
- DOI: 10.1046/j.1365-2567.2003.01711.x
Enhanced mucosal and systemic immune responses to Helicobacter pylori antigens through mucosal priming followed by systemic boosting immunizations
Abstract
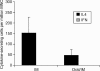
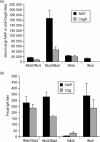
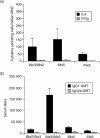
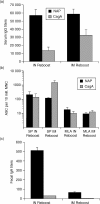
It is estimated that Helicobacter pylori infects the stomachs of over 50% of the world's population and if not treated may cause chronic gastritis, peptic ulcer disease, gastric adenocarcinoma and gastric B-cell lymphoma. The aim of this study was to enhance the mucosal and systemic immune responses against the H. pylori antigens cytotoxin-associated gene A (CagA) and neutrophil-activating protein (NAP), through combinations of mucosal and systemic immunizations in female BALB/c mice. We found that oral or intranasal (i.n.) followed by i.m. immunizations induced significantly higher serum titres against NAP and CagA compared to i.n. alone, oral alone, i.m. alone, i.m. followed by i.n. or i.m. followed by oral immunizations. However, only oral followed by i.m. immunizations induced anti-NAP antibody-secreting cells in the stomach. Moreover, mucosal immunizations alone or in combination with i.m., but not i.m. immunizations alone, induced mucosal immunoglobulin A (IgA) responses in faeces. Any single route or combination of immunization routes with NAP and CagA preferentially induced antigen-specific splenic interleukin-4-secreting cells and far fewer interferon-gamma-secreting cells in the spleen. Moreover, i.n. immunizations alone or in combination with i.m. immunizations induced predominantly serum IgG1 and far less serum IgG2a. Importantly, we found that while both i.n. and i.m. recall immunizations induced similar levels of serum antibody responses, mucosal IgA responses in faeces were only achieved through i.n. recall immunization. Collectively, our data show that mucosal followed by systemic immunization significantly enhanced local and systemic immune responses and that i.n. recall immunization is required to induce both mucosal and systemic memory type responses.
Figures

Similar articles
-
Possible correlates of long-term protection against Helicobacter pylori following systemic or combinations of mucosal and systemic immunizations.Infect Immun. 2007 Jul;75(7):3462-9. doi: 10.1128/IAI.01470-06. Epub 2007 May 14. Infect Immun. 2007. PMID: 17502399 Free PMC article.
-
Oral immunization with Helicobacter pylori-loaded poly(D, L-lactide-co-glycolide) nanoparticles.Helicobacter. 1999 Mar;4(1):33-9. doi: 10.1046/j.1523-5378.1999.09046.x. Helicobacter. 1999. PMID: 10352085
-
Mucosal immune responses are related to reduction of bacterial colonization in the stomach after therapeutic Helicobacter pylori immunization in mice.Microbes Infect. 2006 Feb;8(2):442-9. doi: 10.1016/j.micinf.2005.07.010. Epub 2005 Sep 22. Microbes Infect. 2006. PMID: 16243563
-
The design of vaccines against Helicobacter pylori and their development.Annu Rev Immunol. 2001;19:523-63. doi: 10.1146/annurev.immunol.19.1.523. Annu Rev Immunol. 2001. PMID: 11244046 Review.
-
The role of Th1 and Th2 cells for mucosal IgA responses.Ann N Y Acad Sci. 1996 Feb 13;778:64-71. doi: 10.1111/j.1749-6632.1996.tb21115.x. Ann N Y Acad Sci. 1996. PMID: 8611017 Review.
Cited by
-
Evaluation of mucosal and systemic immune responses elicited by GPI-0100- adjuvanted influenza vaccine delivered by different immunization strategies.PLoS One. 2013 Jul 31;8(7):e69649. doi: 10.1371/journal.pone.0069649. Print 2013. PLoS One. 2013. PMID: 23936066 Free PMC article.
-
Therapeutic vaccination against Helicobacter pylori in the beagle dog experimental model: safety, immunogenicity, and efficacy.Infect Immun. 2004 Jun;72(6):3252-9. doi: 10.1128/IAI.72.6.3252-3259.2004. Infect Immun. 2004. PMID: 15155627 Free PMC article.
-
Immunization of mice with chimeric antigens displaying selected epitopes confers protection against intestinal colonization and renal damage caused by Shiga toxin-producing Escherichia coli.NPJ Vaccines. 2020 Mar 12;5(1):20. doi: 10.1038/s41541-020-0168-7. eCollection 2020. NPJ Vaccines. 2020. PMID: 32194997 Free PMC article.
-
Intramuscular immunization of mice with live influenza virus is more immunogenic and offers greater protection than immunization with inactivated virus.Virol J. 2011 May 21;8:251. doi: 10.1186/1743-422X-8-251. Virol J. 2011. PMID: 21600020 Free PMC article.
-
Production and delivery of Helicobacter pylori NapA in Lactococcus lactis and its protective efficacy and immune modulatory activity.Sci Rep. 2018 Apr 24;8(1):6435. doi: 10.1038/s41598-018-24879-x. Sci Rep. 2018. PMID: 29691472 Free PMC article.
References
-
- Del Giudice G, Covacci A, Telford JL, Montecucco C, Rappuoli R. The design of vaccines against Helicobacter pylori and their development. Annu Rev Immunol. 2001;19:523–63. - PubMed
-
- Marchetti M, Arico B, Burroni D, Figura N, Rappuoli R, Ghiara P. Development of a mouse model of Helicobacter pylori infection that mimics human disease. Science. 1995;267:1655–8. - PubMed
-
- Marchetti M, Rossi M, Giannelli V, et al. Protection against Helicobacter pylori infection in mice by intragastric vaccination with H. pylori antigens is achieved using a non-toxic mutant of E. coli heat-labile enterotoxin (LT) as adjuvant. Vaccine. 1998;16:33–7. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

