Prickle and Strabismus form a functional complex to generate a correct axis during planar cell polarity signaling
- PMID: 12941693
- PMCID: PMC202366
- DOI: 10.1093/emboj/cdg424
Prickle and Strabismus form a functional complex to generate a correct axis during planar cell polarity signaling
Abstract
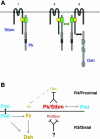
Frizzled (Fz) signaling regulates the establishment of planar cell polarity (PCP). The PCP genes prickle (pk) and strabismus (stbm) are thought to antagonize Fz signaling. We show that they act in the same cell, R4, adjacent to that in which the Fz/PCP pathway is required in the Drosophila eye. We demonstrate that Stbm and Pk interact physically and that Stbm recruits Pk to the cell membrane. Through this interaction, Pk affects Stbm membrane localization and can cause clustering of Stbm. Pk is also known to interact with Dsh and is thought to antagonize Dsh by affecting its membrane localization. Thus our data suggest that the Stbm/Pk complex modulates Fz/Dsh activity, resulting in a symmetry-breaking step during polarity signaling.
Figures





Similar articles
-
The planar cell polarity protein Strabismus promotes Pins anterior localization during asymmetric division of sensory organ precursor cells in Drosophila.Development. 2004 Jan;131(2):469-78. doi: 10.1242/dev.00928. Development. 2004. PMID: 14701683
-
Prickle is phosphorylated by Nemo and targeted for degradation to maintain Prickle/Spiny-legs isoform balance during planar cell polarity establishment.PLoS Genet. 2018 May 14;14(5):e1007391. doi: 10.1371/journal.pgen.1007391. eCollection 2018 May. PLoS Genet. 2018. PMID: 29758044 Free PMC article.
-
Diego and Prickle regulate Frizzled planar cell polarity signalling by competing for Dishevelled binding.Nat Cell Biol. 2005 Jul;7(7):691-7. doi: 10.1038/ncb1271. Epub 2005 Jun 5. Nat Cell Biol. 2005. PMID: 15937478
-
[Molecular mechanisms of epithelial planar polarization].Tanpakushitsu Kakusan Koso. 2005 May;50(6 Suppl):601-7. Tanpakushitsu Kakusan Koso. 2005. PMID: 15926487 Review. Japanese. No abstract available.
-
Van Gogh-like2 (Strabismus) and its role in planar cell polarity and convergent extension in vertebrates.Trends Genet. 2004 Nov;20(11):570-7. doi: 10.1016/j.tig.2004.09.003. Trends Genet. 2004. PMID: 15475117 Review.
Cited by
-
A lateral signalling pathway coordinates shape volatility during cell migration.Nat Commun. 2016 May 26;7:11714. doi: 10.1038/ncomms11714. Nat Commun. 2016. PMID: 27226243 Free PMC article.
-
The kidney and planar cell polarity.Curr Top Dev Biol. 2012;101:185-212. doi: 10.1016/B978-0-12-394592-1.00011-9. Curr Top Dev Biol. 2012. PMID: 23140630 Free PMC article. Review.
-
Notch-dependent Abl signaling regulates cell motility during ommatidial rotation in Drosophila.Cell Rep. 2022 Dec 6;41(10):111788. doi: 10.1016/j.celrep.2022.111788. Cell Rep. 2022. PMID: 36476875 Free PMC article.
-
Modeling bistable cell-fate choices in the Drosophila eye: qualitative and quantitative perspectives.Development. 2010 Jul;137(14):2265-78. doi: 10.1242/dev.044826. Development. 2010. PMID: 20570936 Free PMC article. Review.
-
ECT2 associated to PRICKLE1 are poor-prognosis markers in triple-negative breast cancer.Br J Cancer. 2019 Apr;120(9):931-940. doi: 10.1038/s41416-019-0448-z. Epub 2019 Apr 11. Br J Cancer. 2019. PMID: 30971775 Free PMC article.
References
-
- Adler P.N. (2002) Planar signaling and morphogenesis in Drosophila. Dev. Cell, 2, 525–535. - PubMed
-
- Adler P.N. and Lee,H. (2001) Frizzled signaling and cell–cell interactions in planar polarity. Curr. Opin. Cell Biol., 13, 635–640. - PubMed
-
- Adler P.N., Charlton,J. and Liu,J. (1998) Mutations in the cadherin superfamily member gene dachsous cause a tissue polarity phenotype by altering frizzled signaling. Development, 125, 959–968. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

