Endonucleolytic processing of CCA-less tRNA precursors by RNase Z in Bacillus subtilis
- PMID: 12941704
- PMCID: PMC202377
- DOI: 10.1093/emboj/cdg435
Endonucleolytic processing of CCA-less tRNA precursors by RNase Z in Bacillus subtilis
Abstract
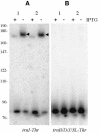
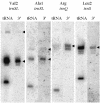
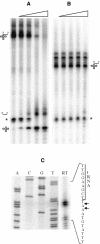
In contrast to Escherichia coli, where the 3' ends of tRNAs are primarily generated by exoribonucleases, maturation of the 3' end of tRNAs is catalysed by an endoribonuclease, known as RNase Z (or 3' tRNase), in many eukaryotic and archaeal systems. RNase Z cleaves tRNA precursors 3' to the discriminator base. Here we show that this activity, previously unsuspected in bacteria, is encoded by the yqjK gene of Bacillus subtilis. Decreased yqjK expression leads to an accumulation of a population of B.subtilis tRNAs in vivo, none of which have a CCA motif encoded in their genes, and YqjK cleaves tRNA precursors with the same specificity as plant RNase Z in vitro. We have thus renamed the gene rnz. A CCA motif downstream of the discriminator base inhibits RNase Z activity in vitro, with most of the inhibition due to the first C residue. Lastly, tRNAs with long 5' extensions are poor substrates for cleavage, suggesting that for some tRNAs, processing of the 5' end by RNase P may have to precede RNase Z cleavage.
Figures





Similar articles
-
RNase Z in Escherichia coli plays a significant role in mRNA decay.Mol Microbiol. 2006 May;60(3):723-37. doi: 10.1111/j.1365-2958.2006.05124.x. Mol Microbiol. 2006. PMID: 16629673
-
Recombinant RNase Z does not recognize CCA as part of the tRNA and its cleavage efficieny is influenced by acceptor stem length.Biol Chem. 2003 Mar;384(3):333-42. doi: 10.1515/BC.2003.039. Biol Chem. 2003. PMID: 12715884
-
Role of metal ions in the hydrolysis reaction catalyzed by RNase P RNA from Bacillus subtilis.J Mol Biol. 1999 Jul 9;290(2):433-45. doi: 10.1006/jmbi.1999.2890. J Mol Biol. 1999. PMID: 10390342
-
The making of tRNAs and more - RNase P and tRNase Z.Prog Mol Biol Transl Sci. 2009;85:319-68. doi: 10.1016/S0079-6603(08)00808-8. Prog Mol Biol Transl Sci. 2009. PMID: 19215776 Review.
-
When all's zed and done: the structure and function of RNase Z in prokaryotes.Nat Rev Microbiol. 2007 Apr;5(4):278-86. doi: 10.1038/nrmicro1622. Nat Rev Microbiol. 2007. PMID: 17363966 Review.
Cited by
-
Degradation of RNA in bacteria: comparison of mRNA and stable RNA.Nucleic Acids Res. 2006 Feb 1;34(2):659-66. doi: 10.1093/nar/gkj472. Print 2006. Nucleic Acids Res. 2006. PMID: 16452296 Free PMC article. Review.
-
Residues in two homology blocks on the amino side of the tRNase Z His domain contribute unexpectedly to pre-tRNA 3' end processing.RNA. 2006 Jun;12(6):1104-15. doi: 10.1261/rna.4206. Epub 2006 Apr 17. RNA. 2006. PMID: 16618969 Free PMC article.
-
Messenger RNA Turnover Processes in Escherichia coli, Bacillus subtilis, and Emerging Studies in Staphylococcus aureus.Int J Microbiol. 2009;2009:525491. doi: 10.1155/2009/525491. Epub 2009 Mar 5. Int J Microbiol. 2009. PMID: 19936110 Free PMC article.
-
Bacterial ribonucleases and their roles in RNA metabolism.Crit Rev Biochem Mol Biol. 2019 Jun;54(3):242-300. doi: 10.1080/10409238.2019.1651816. Crit Rev Biochem Mol Biol. 2019. PMID: 31464530 Free PMC article. Review.
-
Effect of changes in the flexible arm on tRNase Z processing kinetics.J Biol Chem. 2009 Jun 5;284(23):15685-91. doi: 10.1074/jbc.M900745200. Epub 2009 Apr 7. J Biol Chem. 2009. PMID: 19351879 Free PMC article.
References
-
- Castano J.G., Tobian,J.A. and Zasloff,M. (1985) Purification and characterization of an endonuclease from Xenopus laevis ovaries which accurately processes the 3′ terminus of human pre-tRNA-Met(i) (3′ pre-tRNase). J. Biol. Chem., 260, 9002–9008. - PubMed
-
- Deutscher M.P. (1995) tRNA processing nucleases. In Söll,D. and RajBhandary,U.L. (eds), tRNA: Structure, Biosynthesis and Function. American Society for Microbiology, Washington, DC, pp. 51–65.
-
- Deutscher M.P., Lin,J.J. and Evans,J.A. (1977) Transfer RNA metabolism in Escherichia coli cells deficient in tRNA nucleotidyltransferase. J. Mol. Biol., 117, 1081–1094. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

