Group II intron mobility using nascent strands at DNA replication forks to prime reverse transcription
- PMID: 12941706
- PMCID: PMC202375
- DOI: 10.1093/emboj/cdg433
Group II intron mobility using nascent strands at DNA replication forks to prime reverse transcription
Abstract
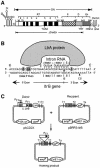
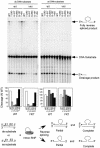
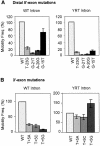
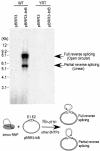
The Lactococcus lactis Ll.LtrB group II intron uses a major retrohoming mechanism in which the excised intron RNA reverse splices into one strand of a DNA target site, while the intron-encoded protein uses a C-terminal DNA endonuclease domain to cleave the opposite strand and then uses the cleaved 3' end as a primer for reverse transcription of the inserted intron RNA. Here, experiments with mutant introns and target sites indicate that Ll.LtrB can retrohome without second-strand cleavage by using a nascent strand at a DNA replication fork as the primer for reverse transcription. This mechanism connecting intron mobility to target DNA replication may be used by group II intron species that encode proteins lacking the C-terminal DNA endonuclease domain and for group II intron retrotransposition to ectopic sites.
Figures

Similar articles
-
Atomic force microscopy reveals DNA bending during group II intron ribonucleoprotein particle integration into double-stranded DNA.Biochemistry. 2006 Oct 17;45(41):12424-35. doi: 10.1021/bi060612h. Biochemistry. 2006. PMID: 17029398 Free PMC article.
-
Characterization of the C-terminal DNA-binding/DNA endonuclease region of a group II intron-encoded protein.J Mol Biol. 2002 Dec 13;324(5):933-51. doi: 10.1016/s0022-2836(02)01147-6. J Mol Biol. 2002. PMID: 12470950
-
Retrotransposition strategies of the Lactococcus lactis Ll.LtrB group II intron are dictated by host identity and cellular environment.Mol Microbiol. 2005 Apr;56(2):509-24. doi: 10.1111/j.1365-2958.2005.04554.x. Mol Microbiol. 2005. PMID: 15813740
-
Group II introns and expression of conjugative transfer functions in lactic acid bacteria.Antonie Van Leeuwenhoek. 1999 Jul-Nov;76(1-4):77-88. Antonie Van Leeuwenhoek. 1999. PMID: 10532373 Review.
-
Bacterial group II introns and their association with mobile genetic elements.Front Biosci. 2002 Aug 1;7:d1843-56. doi: 10.2741/klein1. Front Biosci. 2002. PMID: 12133822 Review.
Cited by
-
Group II introns in eubacteria and archaea: ORF-less introns and new varieties.RNA. 2008 Sep;14(9):1704-13. doi: 10.1261/rna.1056108. Epub 2008 Aug 1. RNA. 2008. PMID: 18676618 Free PMC article.
-
The retrohoming of linear group II intron RNAs in Drosophila melanogaster occurs by both DNA ligase 4-dependent and -independent mechanisms.PLoS Genet. 2012;8(2):e1002534. doi: 10.1371/journal.pgen.1002534. Epub 2012 Feb 16. PLoS Genet. 2012. PMID: 22359518 Free PMC article.
-
A bacterial group II intron-encoded reverse transcriptase localizes to cellular poles.Proc Natl Acad Sci U S A. 2005 Nov 8;102(45):16133-40. doi: 10.1073/pnas.0507057102. Epub 2005 Sep 26. Proc Natl Acad Sci U S A. 2005. PMID: 16186487 Free PMC article.
-
A mutant screen reveals RNase E as a silencer of group II intron retromobility in Escherichia coli.RNA. 2008 Dec;14(12):2634-44. doi: 10.1261/rna.1247608. Epub 2008 Oct 22. RNA. 2008. PMID: 18945808 Free PMC article.
-
Alternative splicing of a group II intron in a surface layer protein gene in Clostridium tetani.Nucleic Acids Res. 2014 Feb;42(3):1959-69. doi: 10.1093/nar/gkt1053. Epub 2013 Nov 8. Nucleic Acids Res. 2014. PMID: 24214997 Free PMC article.
References
-
- Aizawa Y., Xiang,Q., Lambowitz,A.M. and Pyle,A.M. (2003) The pathway for DNA recognition and RNA integration by a group II intron retrotransposon. Mol. Cell, 11, 795–805. - PubMed
-
- Bazaral M. and Helinski,D.R. (1970) Replication of a bacterial plasmid and an episome in Escherichia coli. Biochemistry, 9, 399–406. - PubMed
-
- Belfort M., Derbyshire,V., Parker,M.M., Cousineau,B. and Lambowitz,A.M. (2002) Mobile introns: pathways and proteins. In Craig,N.L., Craigie,R., Gellert,M. and Lambowitz,A.M. (eds), Mobile DNA II. ASM Press Publishers, Washington, DC, pp. 761–783.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

