Phase I/II study of daily carboplatin, 5-fluorouracil and concurrent radiation therapy for locally advanced non-small-cell lung cancer
- PMID: 12942108
- PMCID: PMC2394482
- DOI: 10.1038/sj.bjc.6601227
Phase I/II study of daily carboplatin, 5-fluorouracil and concurrent radiation therapy for locally advanced non-small-cell lung cancer
Abstract
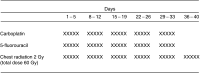
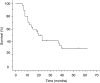
A study was undertaken to determine the maximum tolerated dose, the dose-limiting toxicities and the response rate of carboplatin and 5-fluorouracil administered daily with concurrent thoracic radiation therapy in patients with locally advanced non-small-cell lung cancer. In a phase I/II clinical trial, patients with histologically documented, unresectable stage IIIA or IIIB non-small-cell lung cancer (NSCLC) were enrolled. Carboplatin (20-40 mg m(-2) 2-h infusion, daily) and 5-fluorouracil (200 mg m(-2) 24-h continuous infusion, daily) were administered concurrently with radiotherapy on days 1-33. Radiotherapy, with a total dose of 60 Gy, was delivered in 30 fractions on days 1-40. In the phase I portion, the daily dose of carboplatin was escalated from 20 to 40 mg m(-2). Once the maximum tolerated dose (MTD) and recommended dose (RD) of carboplatin was determined, the study entered the phase II portion. In the phase I portion, the daily MTD and RD of carboplatin were 40 and 35 mg m(-2), respectively. The dose-limiting toxicity was neutropenia. In the following phase II study, 30 patients were entered and the objective response rate was 76.7% (95% CI, 62-92%) and the local control rate was 85.7%. The median survival time was 19.8 months, with a survival rate of 70% at 1 year, 36.7% at 2 years. The major toxicities of treatment were neutropenia (>or=grade 3, 87.9%) and thrombocytopenia (>or=grade 3, 23.3%). This combined therapy for locally advanced non-small-cell lung cancer is promising and shows acceptable toxicity.
Figures
Similar articles
-
Phase I/II study of docetaxel and cisplatin with concurrent thoracic radiation therapy for locally advanced non-small-cell lung cancer.Br J Cancer. 2003 Sep 1;89(5):795-802. doi: 10.1038/sj.bjc.6601217. Br J Cancer. 2003. PMID: 12942107 Free PMC article. Clinical Trial.
-
Dose-escalation study of weekly irinotecan and daily carboplatin with concurrent thoracic radiotherapy for unresectable stage III non-small cell lung cancer.Br J Cancer. 2002 Jul 29;87(3):258-63. doi: 10.1038/sj.bjc.6600464. Br J Cancer. 2002. PMID: 12177791 Free PMC article.
-
A Phase I/II Study of Biweekly Carboplatin and Nab-paclitaxel With Concurrent Radiotherapy for Patients With Locally Advanced Unresectable Stage III Non-small-cell Lung Cancer.Clin Lung Cancer. 2021 Jan;22(1):42-48. doi: 10.1016/j.cllc.2020.09.016. Epub 2020 Oct 14. Clin Lung Cancer. 2021. PMID: 33158764 Clinical Trial.
-
Dose escalation of accelerated hypofractionated three-dimensional conformal radiotherapy (at 3 Gy/fraction) with concurrent vinorelbine and carboplatin chemotherapy in unresectable stage III non-small-cell lung cancer: a phase I trial.Radiat Oncol. 2013 Aug 17;8(1):201. doi: 10.1186/1748-717X-8-201. Radiat Oncol. 2013. PMID: 23957889 Free PMC article. Clinical Trial.
-
Combined-modality therapy for advanced non-small cell lung cancer: paclitaxel and thoracic irradiation.Semin Oncol. 1995 Dec;22(6 Suppl 15):38-44. Semin Oncol. 1995. PMID: 8643969 Review.
Cited by
-
Clinical and dosimetric factors of radiation-induced esophageal injury: radiation-induced esophageal toxicity.World J Gastroenterol. 2005 May 7;11(17):2626-9. doi: 10.3748/wjg.v11.i17.2626. World J Gastroenterol. 2005. PMID: 15849822 Free PMC article.
-
Systemic 5-fluorouracil treatment causes a syndrome of delayed myelin destruction in the central nervous system.J Biol. 2008;7(4):12. doi: 10.1186/jbiol69. Epub 2008 Apr 22. J Biol. 2008. PMID: 18430259 Free PMC article.
-
Deletion of proapoptotic Puma selectively protects hematopoietic stem and progenitor cells against high-dose radiation.Blood. 2010 Jun 10;115(23):4707-14. doi: 10.1182/blood-2009-10-248872. Epub 2010 Apr 1. Blood. 2010. PMID: 20360471 Free PMC article.
References
-
- Arriagada R, Le Chevalier T, Quoix E, Ruffie P, de Cremoux H, Douillard JY, Tarayre M, Pignon JP, Laplanche A (1991) ASTRO (American Society for Therapeutic Radiology and Oncology) plenary: effect of chemotherapy on locally advanced non-small-cell lung carcinoma: a randomized study of 353 patients. GETCB (Groupe d'Etude et Traitement des Cancers Bronchiques), FNCLCC (Federation Nationale des Centres de Lutte contre le Cancer) and the CEBI trialists. Int J Radiat Oncol Biol Phys 20: 1183–1190 - PubMed
-
- Begg AC, van der Kolk PJ, Emondt J, Bartelink H (1987) Radiosensitization in vitro by cis-diammine (1,1-cyclobutanedicarboxylato) platinum(II) (carboplatin, JM8) and ethylenediammine-malonatoplatinum(II) (JM40). Radiother Oncol 9: 157–165 - PubMed
-
- Belani CP, Aisner J (1994) Combined chemotherapy and radiation in locally advanced non-small cell lung cancer. Semin Oncol 21: 79–90 - PubMed
-
- Belani CP, Aisner J, Bahri S, Jett J, Day R, Capazolli MJ, Hiponia D, Engstrom C (1996) Chemoradiotherapy in non-small cell lung cancer: paclitaxel/carboplatin/radiotherapy in regionally advanced disease. Semin Oncol 23: 113–116 - PubMed
-
- Blackstock AW, Lesser GJ, Fletcher-Steede J, Case LD, Tucker RW, Russo SM, White DR, Miller A (2001) Phase I study of twice-weekly gemcitabine and concurrent thoracic radiation for patients with locally advanced non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 51: 1281–1289 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous